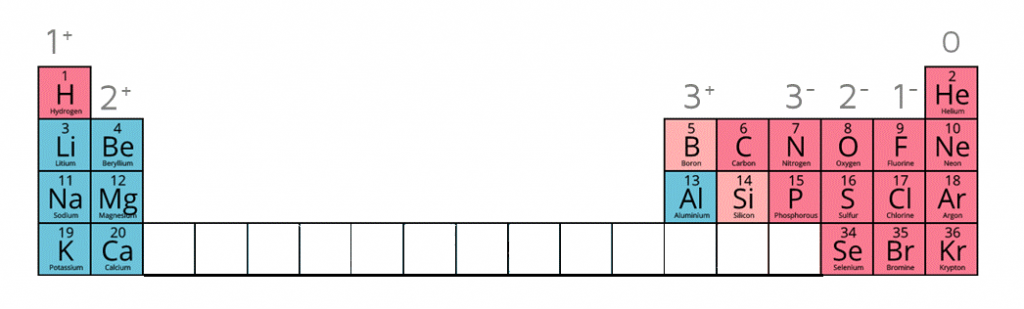
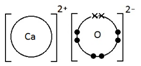1:37 understand how ions are formed by electron loss or gain
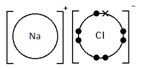
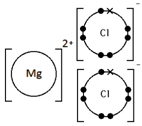
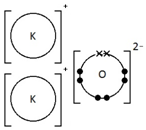
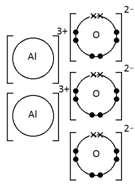
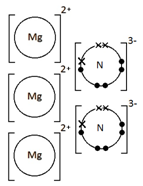
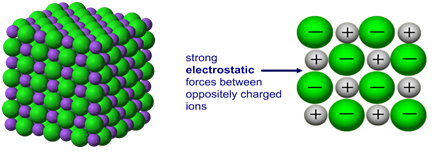
Ions are electrically charged particles formed when atoms lose or gain electrons.
They have the same electronic structures as noble gases.
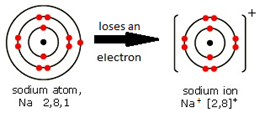
Metal atoms form positive ions (cations).
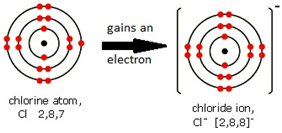
Non-metal atoms form negative ions (anions).