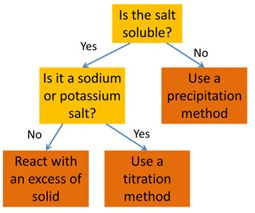
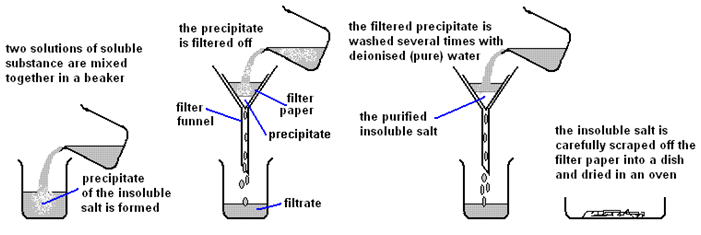
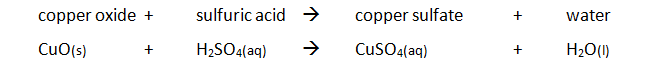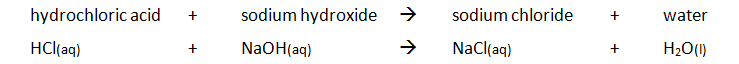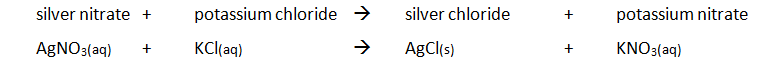Acid reactions summary
alkali + acid → water + salt
base + acid → water + salt
carbonate + acid → water + salt + carbon dioxide
metal + acid → salt + hydrogen
To assist remembering this list, many pupils find it useful to remember this horrid looking but very effective mnemonic:
AAWS
BAWS
CAWS CoD
MASH
Acids are a source of hydrogen ions (H⁺) when in solution. When the hydrogen in an acid is replaced by a metal, the compound is called a salt. The name of the salt depends on the acid used. For example if sulfuric acid is used then a sulfate salt will be formed.
| Parent acid | Formula | Salt | Formula ion |
| sulfuric acid | H2SO4 | sulfate | SO42- |
| hydrochloric acid | HCl | chloride | Cl- |
| nitric acid | HNO3 | nitrate | NO3- |
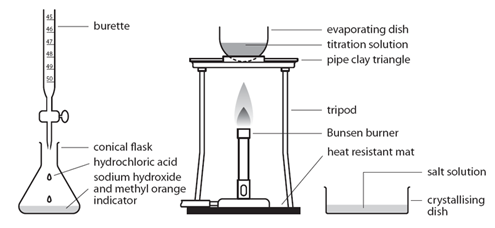
Acid + Alkali and Acid + Base
A base is a substance that can neutralise an acid, forming a salt and water only.
Alkalis are soluble bases. When they react with acids, a salt and water is formed. The salt formed is often as a colourless solution. Alkalis are a source of hydroxide ions (OH⁻) when in solution.
alkali + acid → water + salt
base + acid → water + salt
Examples of acid + alkali reactions:
- sodium hydroxide + hydrochloric acid → sodium chloride + water
- NaOH (aq) + HCl (aq) → NaCl (aq) + H₂O (l)
- potassium hydroxide + sulfuric acid → potassium sulfate + water
- 2KOH (aq) + H₂SO₄ (aq) → K₂SO₄ (aq) + 2H₂O (l)
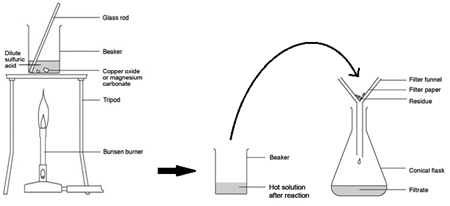
Example of an acid + base reaction:
CuO (s) + H₂SO₄ (aq) → CuSO₄ (aq) + H₂O (l)
Acid + Carbonate
carbonate + acid → water + salt + carbon dioxide
A carbonate is a compound made up of metal ions and carbonate ions. Examples of metal carbonates are sodium carbonate, copper carbonate and magnesium carbonate.
When carbonates react with acids, bubbling is observed which is the carbon dioxide being produced. If the acid is in excess the carbonate will disappear.
Examples of acid + carbonate reactions:
- calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide
- CaCO₃ (s) + 2HCl (aq) → CaCl₂ (aq) + H₂O (l) + CO₂ (g)
- potassium carbonate + hydrochloric acid → potassium chloride + water + carbon dioxide
- K₂CO₃ (aq) + 2HCl (aq) → 2KCl (aq) + H₂O (l) + CO₂ (g)
Acid + Metal
metal + acid → salt + hydrogen
Metals will react with an acid if the metal is above hydrogen in the reactivity series.
When metals react with acids, bubbling is observed which is the hydrogen being produced. If the acid is in excess the metal will disappear.
Examples of acid + metal reactions:
- magnesium + sulfuric acid → magnesium sulfate + hydrogen
- Mg (s) + H₂SO₄ (aq) → MgSO₄ (aq) + H₂ (g)
- aluminium + hydrochloric acid → aluminium chloride + hydrogen
- 2Al (s) + 6HCl (aq) → 2AlCl₃ (aq) + 3H₂ (g)
- copper + hydrochloric acid → no reaction (since copper is below hydrogen in the reactivity series)