4:23 know that alkenes contain the functional group >C=C<
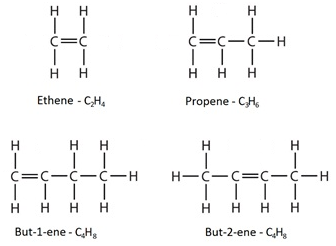
Alkenes are a homologous series of hydrocarbons which contain a carbon-carbon double bond. This double bond is shown in formulae as a double line.
The names of alkenes end with “ene”.
An example is ethene, the structural formula for which is CH₂ = CH₂
For a molecule with more than two carbon atoms, the position of the double bond within the molecule can vary as indicated by the name and the structural formula.