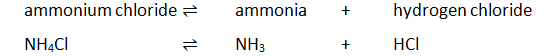3:18 describe reversible reactions such as the dehydration of hydrated copper(II) sulfate and the effect of heat on ammonium chloride
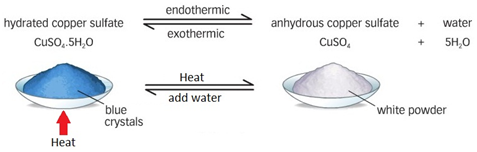
Dehydration of copper(II) sulfate
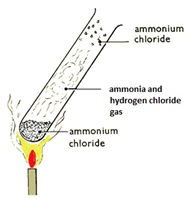 Heating ammonium chloride
Heating ammonium chloride
On heating, white solid ammonium chloride decomposes forming ammonia and hydrogen chloride gas. On cooling, ammonia and hydrogen chloride react to form a white solid of ammonium chloride: