4:02 understand how to represent organic molecules using empirical formulae, molecular formulae, general formulae, structural formulae and displayed formulae
The molecular formula shows the actual number of atoms of each element in a molecule.
The empirical formula shows the simplest whole number ratio of atoms present in a compound. So the molecular formula is a multiple of the empirical formula.
The general formula shows the relationship between the number of atoms of one element to another within a molecule. Members of a homologous series share the same general formula. The general formula for alkanes is CnH2n+2 and the general formula for alkenes is CnH2n.
A structural formula shows how the atoms in a molecule are joined together.
The displayed formula is a full structural formula which shows all the bonds in a molecule as individual lines.
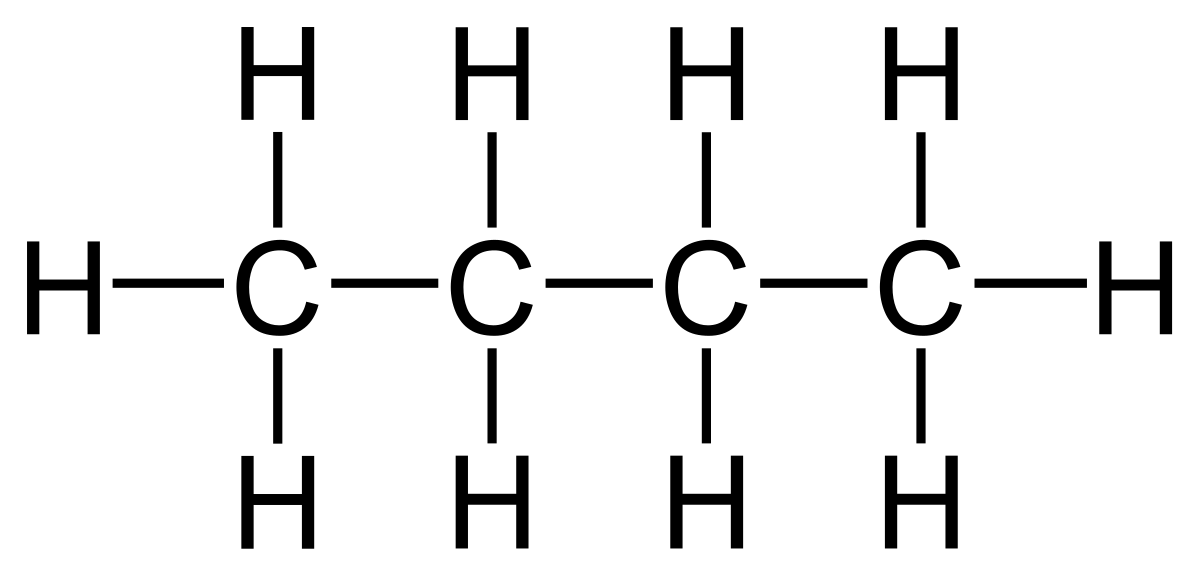
The terms above are demonstrated with the example of butane.
- Displayed formula:
- Molecular formula: C₄H₁₀
- Empirical formula: C₂H₅
- General formula (alkanes): CnH2n+2
- Structural formula: CH₃ – CH₂ – CH₂ – CH₃
The terms above are demonstrated with the example of ethene, which contains a double bond.
- Displayed formula:
- Molecular formula: C₂H₄
- Empirical formula: CH₂
- General formula (alkenes): CnH2n
- Structural formula: CH₂ = CH₂