Moles quiz
[WpProQuiz 63]
[WpProQuiz 62]
[WpProQuiz 64]
[WpProQuiz 61]
[WpProQuiz 65]
[WpProQuiz 60]
Example:
Sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
Word equation:
sodium + water –> sodium hydroxide + hydrogen
Writing the chemical equation
A chemical equation represents what happens in terms of atoms in a chemical reaction.
Step 1: To write a chemical equation we need to know the chemical formulae of the substances.
Na + H2O –> NaOH + H2
Step 2: The next step is to balance the equation: write a large number before each compound so the number of atoms of each element on the left hand side (reactants) matches the number on the right (products). This large number is the amount of each compound or element.
During this balancing stage the actual formulas for each compound must not be changed. Only the number of each compound changes.
2Na + 2H2O –> 2NaOH + H2
If asked for an equation, the chemical equation must be given.
State symbols are used to show what physical state the reactants and products are in.
| State symbols | Physical state |
|---|---|
| (s) | Solid |
| (l) | Liquid |
| (g) | Gas |
| (aq) | Aqueous solution (dissolved in water) |
Example:
A solid piece of sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
2Na(s) + 2H2O(l) –> 2NaOH(aq) + H2(g)
This excellent Tyler de Witt video is an introduction to balancing equations:
And here’s another of the lovely Tyler’s videos with some practice questions and answers on equation balancing:
Relative formula mass (Mr) is mass of a molecule or compound (on a scale compared to carbon-12).
It is calculated by adding up the relative atomic masses (Ar) of all the atoms present in the formula.
Example:
The relative formula mass (Mr) for water (H2O) is 18.
Water = H2O
Atoms present = (2 x H) + (1 x O)
Mr = (2 x 1) + (1 x 16) = 18
Here’s an excellent Tyler de Witt video explaining how to calculate the relative formula mass of compounds with:
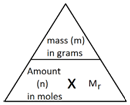
In Chemistry, the mole is a measure of amount of substance (n).
The abbreviation for mole is mol.
The mass of 1 mole of a substance is the relative formula mass (Mr) of the substance in grams.
Example:
The Mr of water is 18.
Therefore the mass of 1 mol of water equals 18 g.
The following formula allows for the interconversion between a mass in grams and a number of moles for a given substance:
Example 1:
Calculate the amount, in moles, of 8.8 g of carbon dioxide (CO2).
Step 1: Calculate the relative formula mass (Mr) of carbon dioxide (CO2).
Step 2: Use the formula to calculate the amount in moles.
Example 2:
Calculate the mass of 2 mol of copper(II) sulfate (CuSO4).
Step 1: Calculate the relative formula mass (Mr) of copper(II) sulfate (CuSO4).
Step 2: Rearrange the formula to calculate the mass.
This video shows how to perform calculations involving mass (in grams), amount (in moles) and relative atomic mass:
Example: When calcium carbonate (CaCO3) is heated calcium oxide is produced. You can use reacting mass calculations to calculate the mass of calcium oxide produced when heating 25 g of calcium carbonate.
CaCO3 –> CaO + CO2
Step 1: Calculate the amount, in moles, of 25 g of calcium carbonate (CaCO3)
Step 2: Deduce the amount, in moles, of CaO produced from 0.25 mol of CaCO3.
This step involves using the ratio of CaCO3 to CaO from the chemical equation.
CaCO3 –> CaO + CO2
From the equation you can see that the ratio of CaCO3 to CaO is 1:1.
Therefore if you have 0.25 mol of CaCO3 this will produced 0.25 mol of CaO.
Step 3: Calculate the mass of 0.25 mol of CaO.
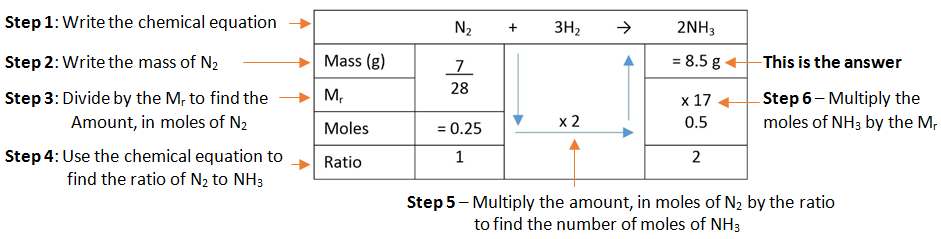
A simple format for laying out this method can be used.
Example: What mass of ammonia (NH3) is formed when 7 g of nitrogen (N2) is combined with hydrogen (H2).
This video steps through a very useful method used to calculate reacting masses.