4:27 describe the reactions of alkenes with bromine, to produce dibromoalkanes
Alkenes react with bromine water. UV light is not required for this reaction.
The double bond is broken and the bromine atoms are added. This is an addition reaction.
During this reaction there is a colour change from orange to colourless.
For example:
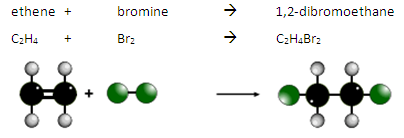
This is how we can test for the presence of an alkene or another type of unsaturated molecule.