2.01 use the following units: ampere (A), coulomb (C), joule (J), ohm (Ω), second (s), volt (V) and watt (W)
unit for:
current : Ampere (A)
charge : coulomb (C)
resistance : ohm (Ω)
time : second (s)
potential difference : volt (V)
power : watt (W)
unit for:
current : Ampere (A)
charge : coulomb (C)
resistance : ohm (Ω)
time : second (s)
potential difference : volt (V)
power : watt (W)
power (w) = current (A) x voltage (V)
when looking at a circuit a component will be given a power and a voltage appropriate to run at then the current can be calculated so the rating of the fuse can be selected for slightly higher than that.
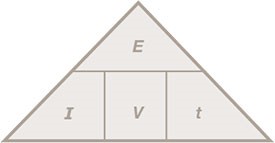
Energy (J) = potential difference (V) x current (A) x Time (s)
Advantages of parallel circuits:
Advantages of series circuits:
Notes on current:
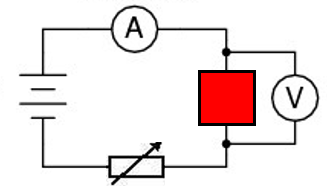
in the bellow diagram the red box could represent a wire, a bulb, a resistor or a diode.
By changing the resistance of the variable resistor the graphs are reproduced.
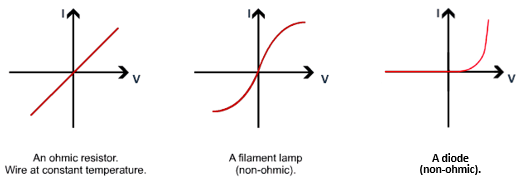
Since V = IR, as you increase the resistance in a circuit, the current will decrease.
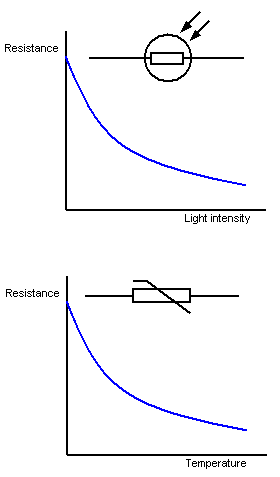
LDR
As illumination increases, resistance decreases
Thermistor
As temperature increases, resistance decreases.
A lamp can be added to a circuit to check for a current. If current is flowing, the lamp will light up.
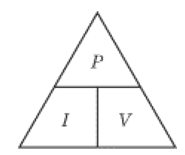
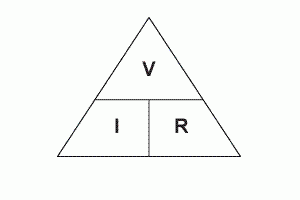
Potential difference (V) = Current (A) x Resistance (Ω)
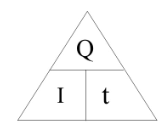
current is rate of flow of charge so I=Q/t
Charge (C) = Current (A) x Time (s)
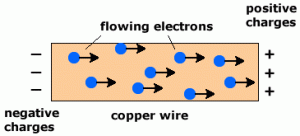
Electrons are negatively charged and free to flow in a metal so carry charge
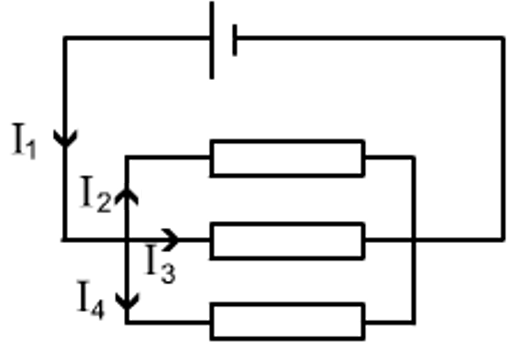
At a junction current ‘splits’ to take both paths.
It comes back together when the paths meet again.
I1 = I2 + I3 +I4
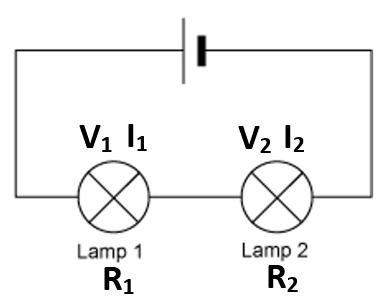
VT = V1 + V2
IT = I1 = I2
RT = R1 + R2
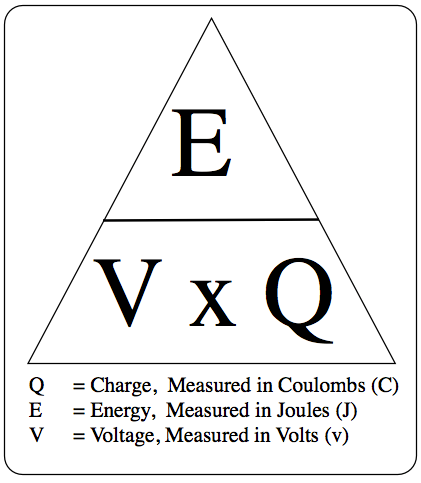
Energy Transferred (J) = charge (C) x Voltage (V)
Conducting Materials:
Will conduct electricity
Insulating Materials:
Will not conduct electricity