Fundamentals quiz
Solid
Arrangement: Particles are close together and regularly packed.
Movement: Particles vibrate around a fixed point.
Energy: Particles have less kinetic energy than both liquids and gasses.
Liquid

Arrangement: Particles are close together but irregular.
Movement: Particles are free to move.
Energy: Particles have less kinetic energy than gasses but more than solids.
Gas
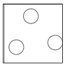
Arrangement: Particles are far apart and there are no forces between them.
Movement: Particles are free to move.
Energy: Particles have more kinetic energy than liquids and solids.
Melting: When a solid is heated, the energy makes the particles vibrate fast enough so that the forces of attraction between the particles break. For example H2O(s) –> H2O(l)
Freezing: When a liquid is cooled, the particles move slow enough so that the forces of attraction between them will hold them into a solid. For example H2O(l) –> H2O(s)
Boiling: When a liquid is heated strongly, the energy makes the particles move fast enough so that all forces of attraction are broken. For example H2O(l) –> H2O(g)
Condensing: When a gas is cooled, the particles move slow enough so that the forces of attraction between them will hold them as a liquid. For example H2O(g) –> H2O(l)
Sublimation: A small number of substances have the ability to change directly from a solid to a gas when heated. For example CO2(s) –> CO2(g)
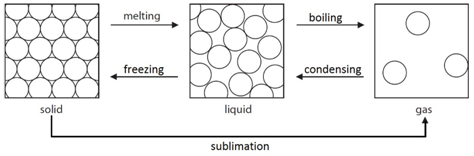
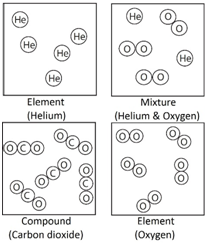
Element: The simplest type of substances made up of only one type of atom.
Compound: A substance that contains two or more elements chemically joined together in fixed proportions.
Mixture: Different substances in the same space, but not chemically combined.
Note: elements such as oxygen (O2) are described as diatomic because they contain two atoms.
The full list of elements that are diatomic is:
Watch Daniel Radcliffe sing the names of all the elements – it’s just a shame there are now more elements than were written into Tom Lehrer’s famous song…..
Pure substances, such as an element or a compound, melt and boil at fixed temperatures.
However, mixtures melt and boil over a range of temperatures.
Example: although pure water boils at 100⁰C, the addition of 10g of sodium chloride (NaCl) to 1000cm³ of water will raise the boiling point to 100.2⁰C.
Example: although pure water melts at 0⁰C, the addition of 10g of sodium chloride (NaCl) to 1000cm³ of water will lower the melting point to -0.6⁰C.
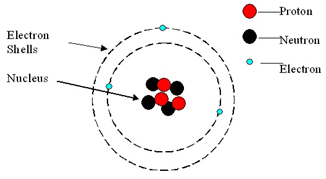
Atom: An atom is the smallest part of an element.
An atom consists of a central nucleus, composed of protons and neutrons.
This is surrounded by electrons, orbiting in shells (energy levels).
Atoms are neutral because the numbers of electrons and protons are equal.
| Mass | Charge | |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | negligible (1/1836) | -1 |
This video explains the basics of atomic structure, telling you what is inside an atom:
This is a good interactive demonstration showing how subatomic particles make up the atoms in the Periodic Table.
Atomic number: The number of protons in an atom.
Mass number: The number of protons and neutrons in an atom.
Relative atomic mass (Ar): The average mass of an atom compared to 1/12th the mass of carbon-12.
The elements in the Periodic Table are arranged in order of increasing atomic number.
Columns are called Groups. They indicate the number of electrons in the outer shell of an atom.
Rows are called Periods. They indicate the number of shells (energy levels) in an atom.
Metals
Non – Metals
Metals on the left of the Periodic Table.
Non-Metals on the top-right, plus Hydrogen.
Example:
Sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
Word equation:
sodium + water –> sodium hydroxide + hydrogen
Writing the chemical equation
A chemical equation represents what happens in terms of atoms in a chemical reaction.
Step 1: To write a chemical equation we need to know the chemical formulae of the substances.
Na + H2O –> NaOH + H2
Step 2: The next step is to balance the equation: write a large number before each compound so the number of atoms of each element on the left hand side (reactants) matches the number on the right (products). This large number is the amount of each compound or element.
During this balancing stage the actual formulas for each compound must not be changed. Only the number of each compound changes.
2Na + 2H2O –> 2NaOH + H2
If asked for an equation, the chemical equation must be given.
State symbols are used to show what physical state the reactants and products are in.
| State symbols | Physical state |
|---|---|
| (s) | Solid |
| (l) | Liquid |
| (g) | Gas |
| (aq) | Aqueous solution (dissolved in water) |
Example:
A solid piece of sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
2Na(s) + 2H2O(l) –> 2NaOH(aq) + H2(g)
This excellent Tyler de Witt video is an introduction to balancing equations:
And here’s another of the lovely Tyler’s videos with some practice questions and answers on equation balancing:
This is useful to help you to practice how to balance equations:
Relative formula mass (Mr) is mass of a molecule or compound (on a scale compared to carbon-12).
It is calculated by adding up the relative atomic masses (Ar) of all the atoms present in the formula.
Example:
The relative formula mass (Mr) for water (H2O) is 18.
Water = H2O
Atoms present = (2 x H) + (1 x O)
Mr = (2 x 1) + (1 x 16) = 18
Here’s an excellent Tyler de Witt video explaining how to calculate the relative formula mass of compounds with:
Exothermic: chemical reaction in which heat energy is given out.
Endothermic: chemical reaction in which heat energy is taken in.
(So, in an exothermic reaction the heat exits from the chemicals so temperature rises)