5.08 explain why heating a system will change the energy stored within the system and raise its temperature or produce changes of state
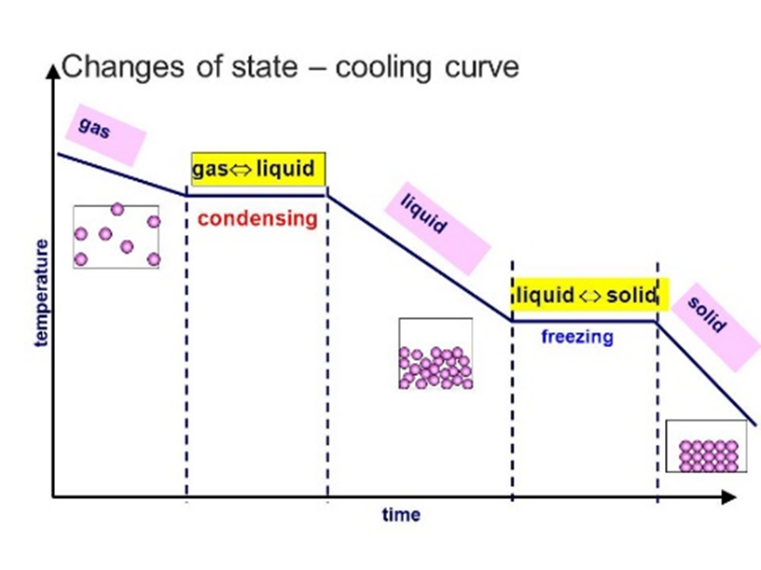
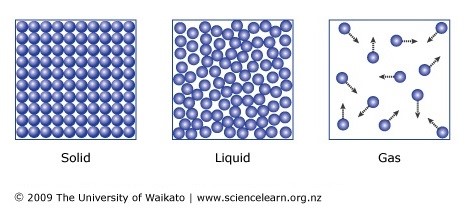
solids:
liquids:
gasses:
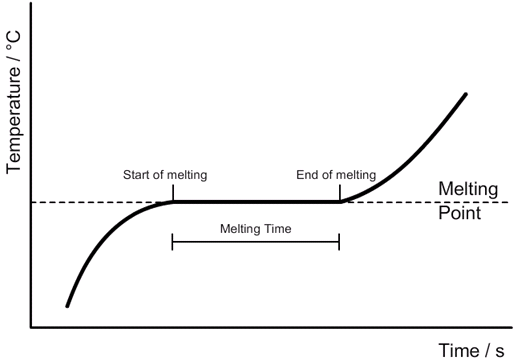
Specific heat capacity:
Change in thermal energy [J] = Mass [kg] x Specific heat capacity [J/kg 0C] x Change in temperature [0C]