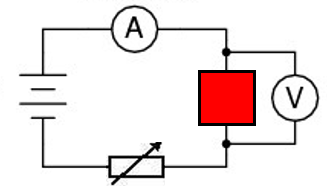
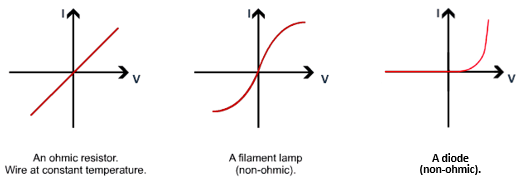
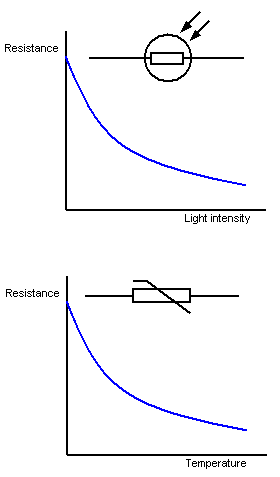
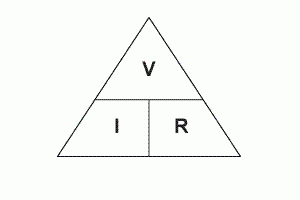
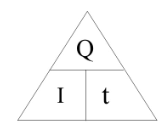
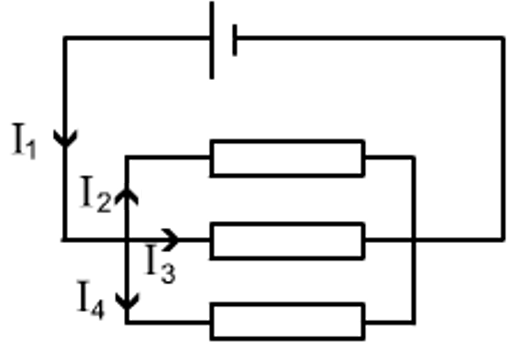
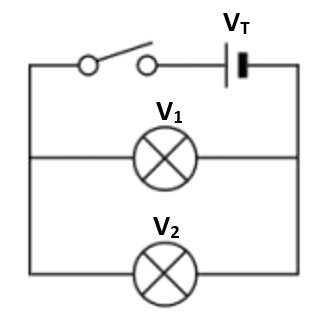
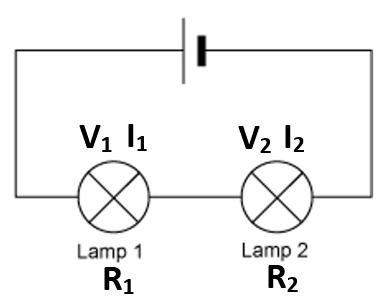
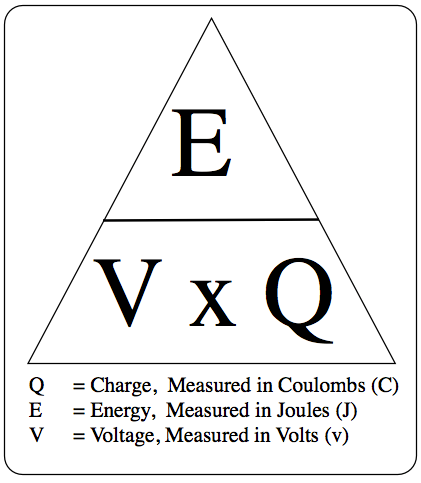
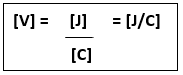2.07 explain why a series or parallel circuit is more appropriate for particular applications, including domestic lighting
Advantages of parallel circuits:
- Components (e.g. bulbs) may be switched on/off independently.
- If one component breaks, current can still flow through the other parts of the circuit.
- Bulbs maintain a similar brightness.
Advantages of series circuits:
- Fewer wires, cheaper and easier to assemble.
- Uses less power