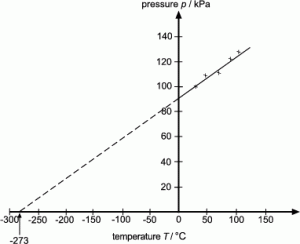
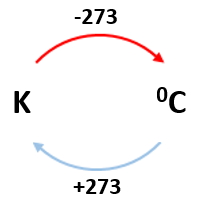
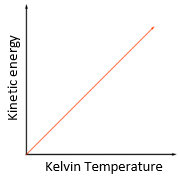
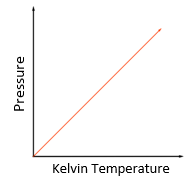
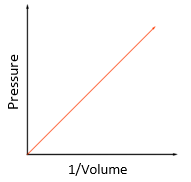
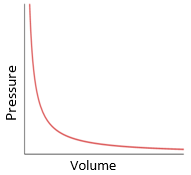
5.15 explain how molecules in a gas have random motion and that they exert a force and hence a pressure on the walls of a container
Gas laws:
- Gas molecules have rapid and random motion.
- When they hit the walls of the container, they exert a force.
- Pressure = Force/Area