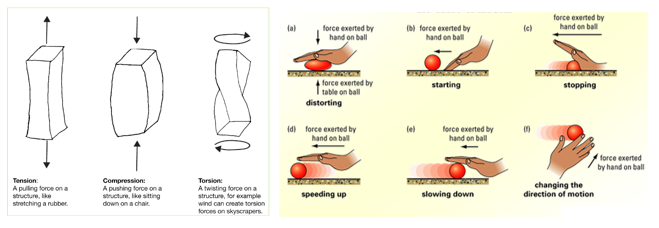
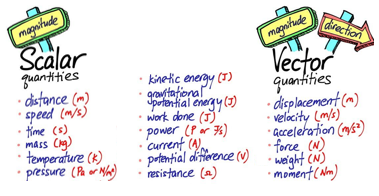
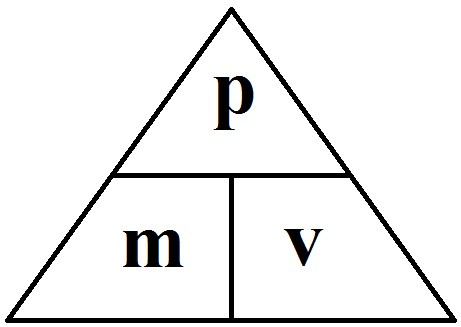
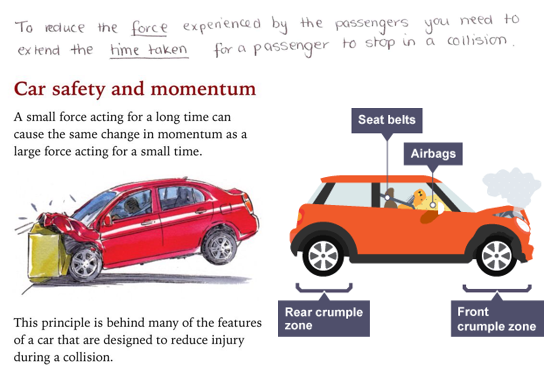
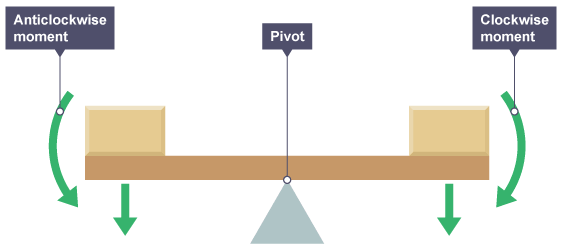
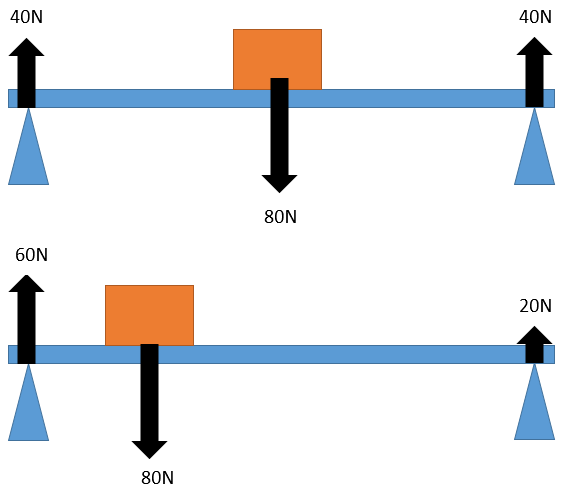
1.01 use the following units: kilogram (kg), metre (m), metre/second (m/s), metre/second^2(m/s^2), newton (N), second (s) and newton/kilogram(N/kg)
Make sure you are familiar with units for
Mass: kilogram (kg)
Distance: metre (m)
Speed: metre per second (m/s)
Acceleration: metre per second squared (m/s^2)
Force: newton (N)
Time: second (s)
Gravity: newton/kilogram (N/kg)