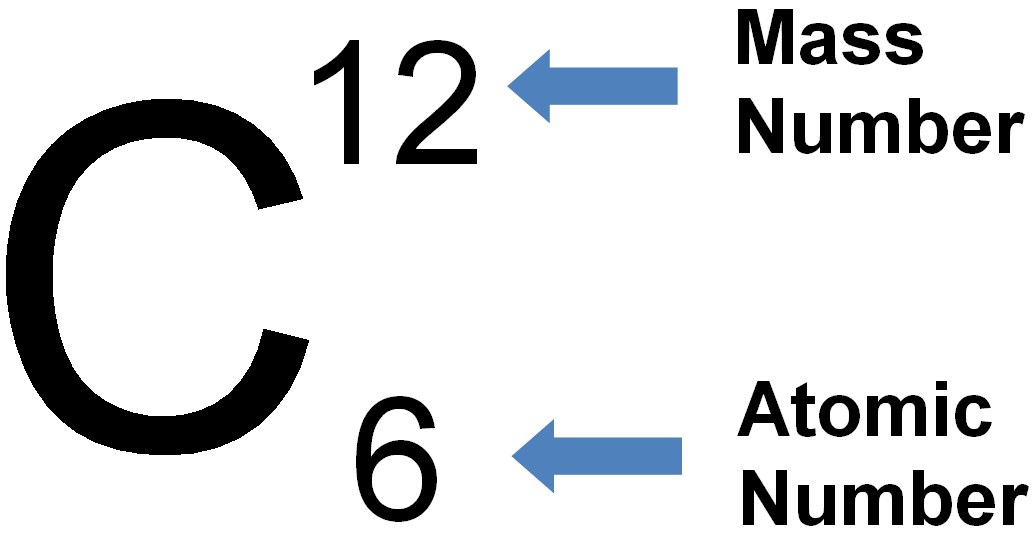
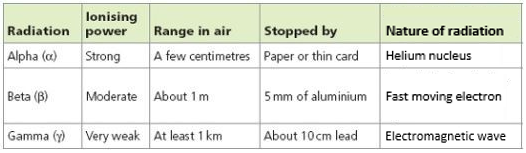
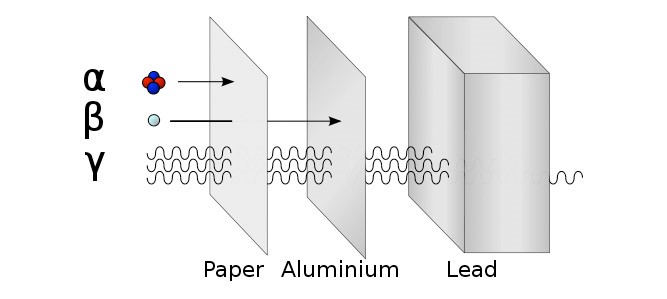
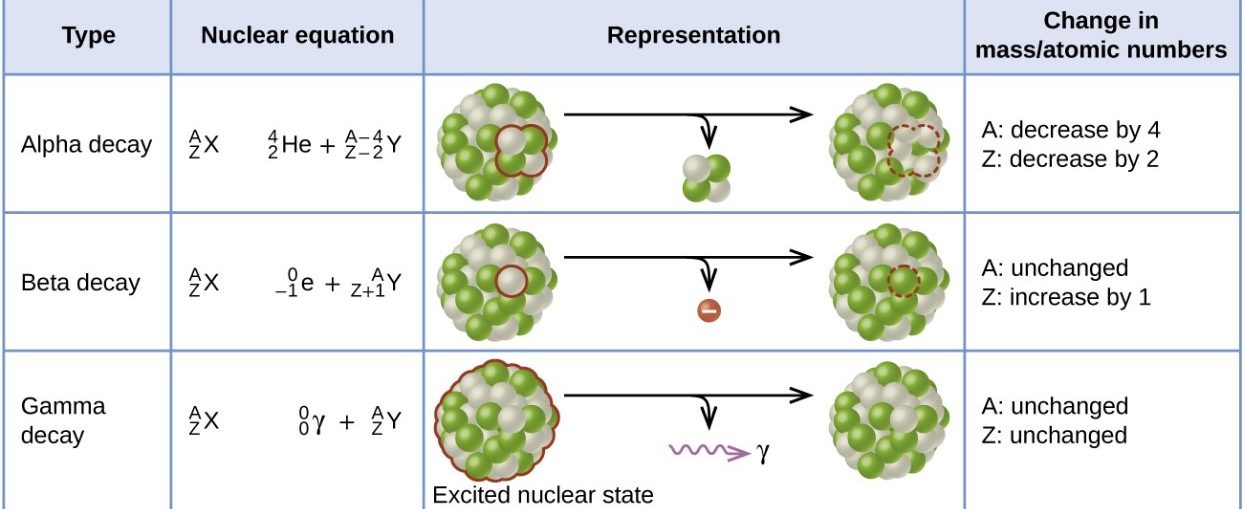
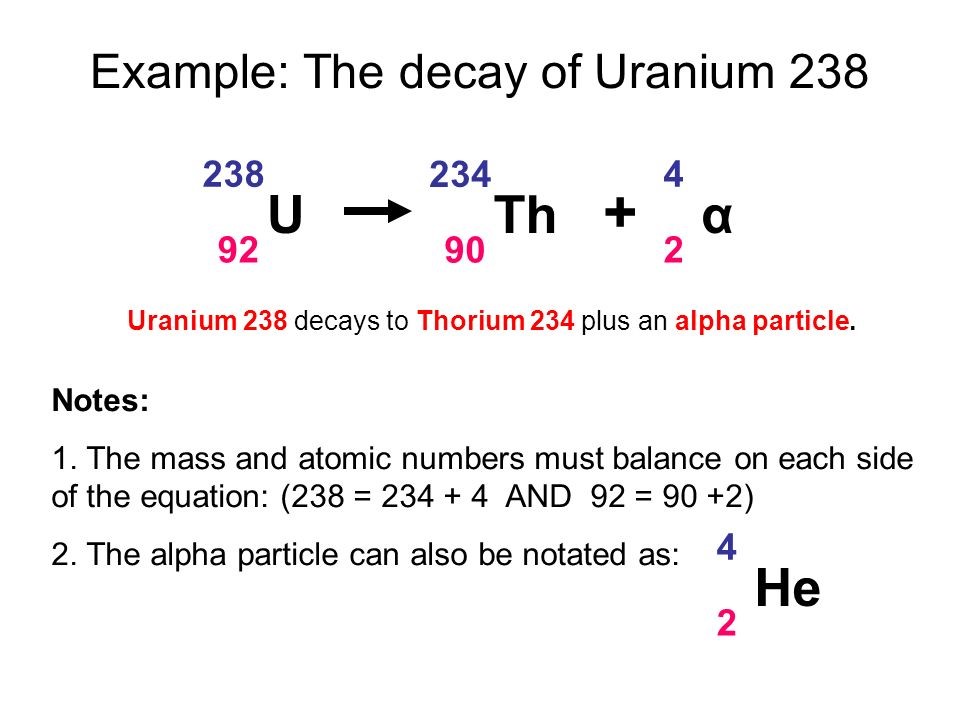
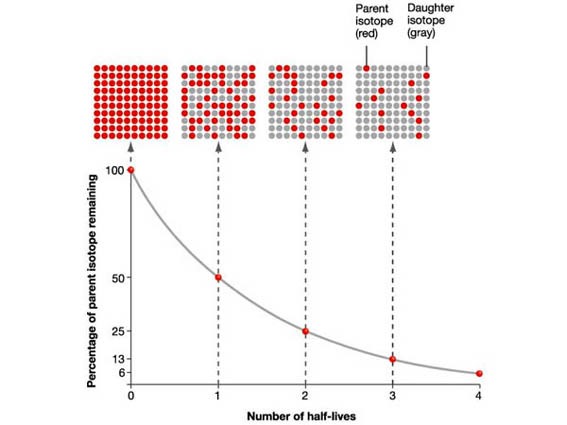
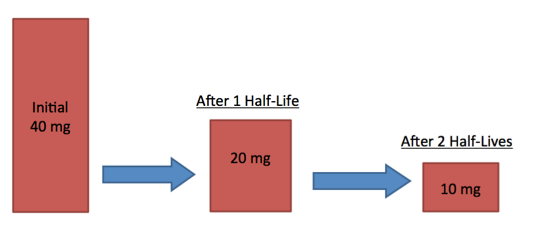
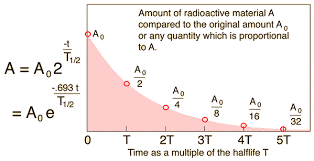
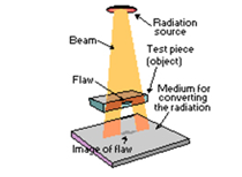
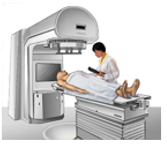
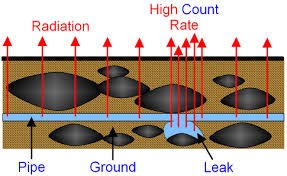
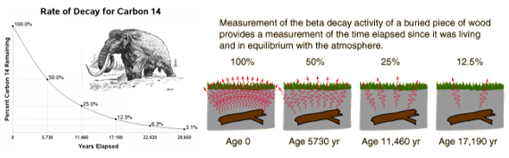
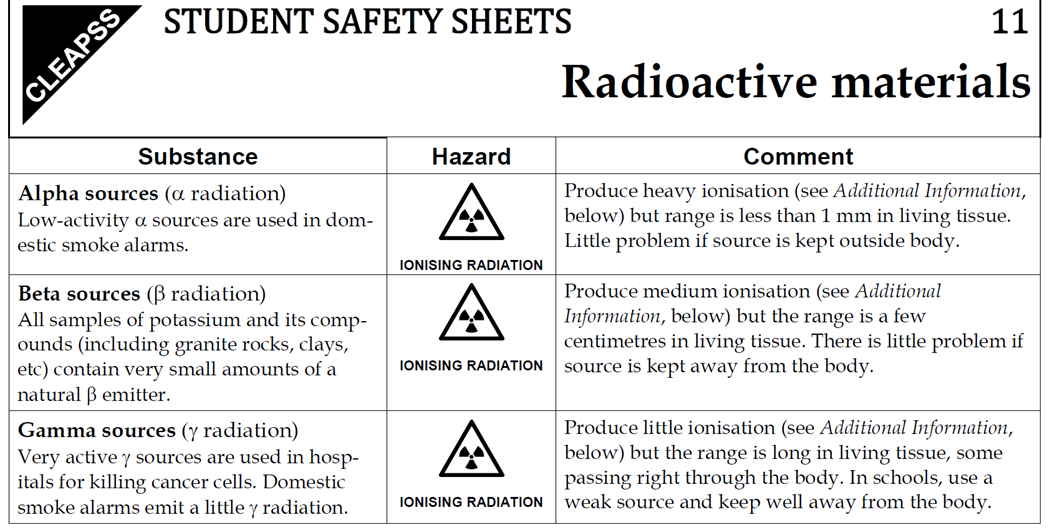
7.02 describe the structure of an atom in terms of protons, neutrons and electrons and use symbols such as 146C to describe particular nuclei
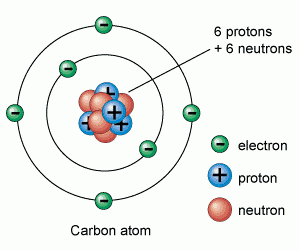
Atoms are made up of protons, neutrons and electrons.
Protons and neutrons are in the nucleus, electrons are in the shells