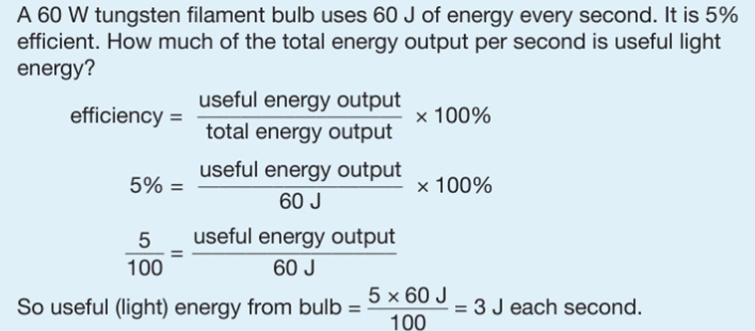
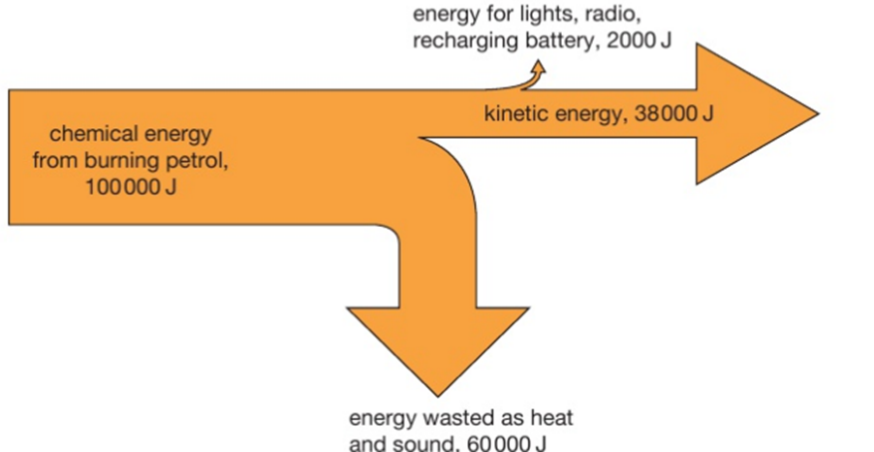
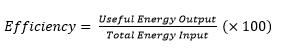4.01 use the following units: kilogram (kg), joule (J), metre (m), metre/second (m/s), metre/second2 (m/s2), newton (N), second (s) and watt (W)
know the units for
Mass = kilogram (kg)
energy = joule (J)
velocity = metre/second (m/s)
acelleration = metre/ second 2 (m/s2)
force = newton (N)
time = second (s)
power = watt (W)