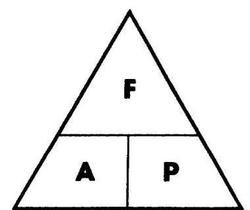
5.03 know and use the relationship between density, mass and volume:
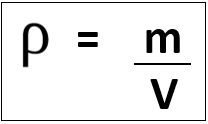
Units of density depend on units used for mass and volume:
E.g. mass in [g] and volume in [cm3] gives density in [g/cm3], however mass in [kg] and volume in [m3] gives density in [kg/m3].