Titration Method:
Preparing pure dry crystals of sodium chloride (NaCl) from hydrochloric acid (HCl) and sodium hydroxide (NaOH)
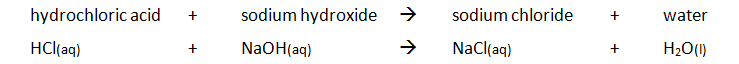
Before the salt preparation is carried out using the below method, the volume of acid that exactly reacts with 25cm3 of the alkali is found by titration using methyl orange indicator.
| Step | Explanation |
|---|---|
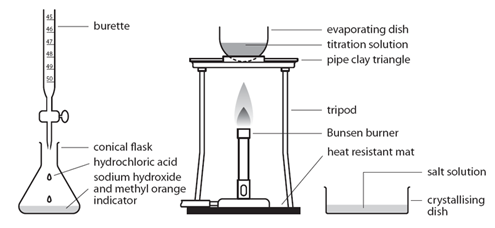
| Pipette 25cm3 of alkali (NaOH) into a conical flask | Accurately measures the alkali (NaOH) |
| Do not add indicator | Prevents contamination of the pure crystals with indicator |
| Using the titration values, titrate the known volume acid (HCl) into conical flask containing alkali | Exactly neutralises all of the alkali (NaOH) |
| Transfer to an evaporating basin & heat the solution | Forms a hot saturated solution (NaCl(aq)) |
| Allow the solution to cool so that hydrated crystals form | Sodium chloride is less soluble in cold water |
| Remove the crystals by filtration and wash with distilled water | Removes any impurities |
| Dry by leaving in a warm place | Evaporates the water |
(Note – This process could be reversed with the acid in the pipette and the alkali in the burette)
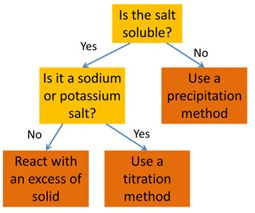
How to select the right method for preparing a salt: