Structure & Bonding (Triple) quiz
This should give you a ruff idea of what the difference is between ionic and covalent bonding.
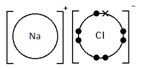
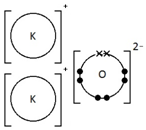
Sodium chloride, NaCl
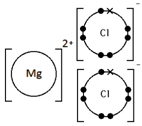
Calcium oxide, CaO
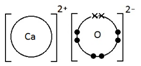
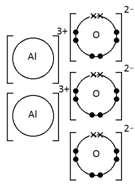
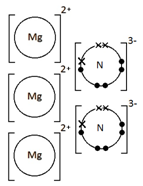
Ionic bonding: a strong electrostatic attraction between oppositely charged ions.
Ionic compounds have high melting and boiling points because they have a giant structure with strong electrostatic forces between oppositely charged ions that require a lot of energy to break.
Giant 3D lattice of sodium chloride (NaCl)
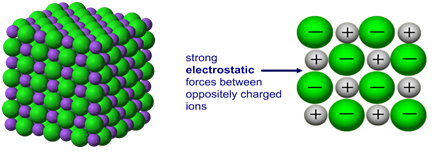
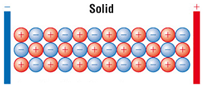
Ionic compounds do not conduct electricity when solid.
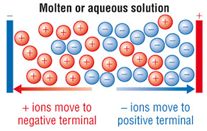
However, ionic compounds do conduct electricity if molten or in solution.
These videos are a really good introduction to ionic bonding:
A covalent bond is formed between two non-metal atoms by sharing a pair of electrons in order to fill the outer shell.
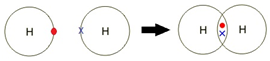
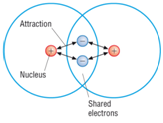
Covalent bonding: a strong attraction between a shared pair of electrons and two nuclei.
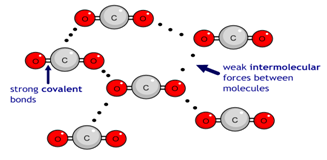
Carbon dioxide (CO2) has a simple molecular structure. This just means that it is made up of molecules.
Within each molecule are atoms bonded to each other covalently. These covalent bonds inside the molecules are strong.
However, between the molecules are weak forces of attraction that require little energy to break. These forces are not covalent bonds. This is why simple molecular substances such as carbon dioxide have a low boiling point.
So when carbon dioxide changes from a solid to a gas, for example, that process can be represented as:
CO₂ (s) → CO₂ (g)
Notice that even though there has been a dramatic change of state from solid to gas, the substance before and after the change is always made up of carbon dioxide molecules. During the change of the state the covalent bonds within each molecule remain unbroken. Instead it is the weak forces of attraction between the molecules which have been overcome.
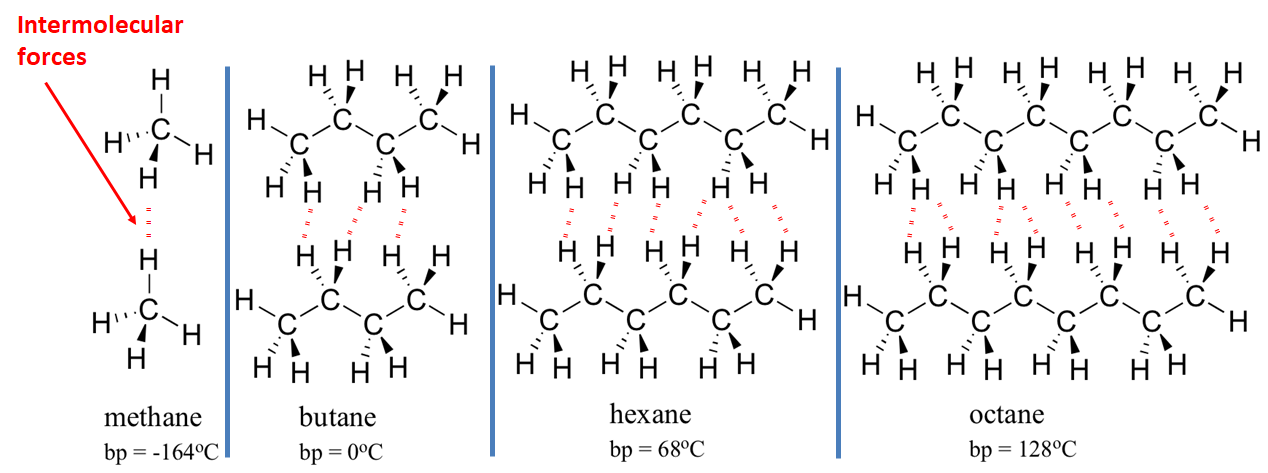
Larger molecules tend to have higher boiling points.
This is because larger molecules (molecules with more mass) have more forces of attraction between them. These forces, although weak, must be overcome if the substance is to boil, and larger molecules have more attractions which must be overcome.
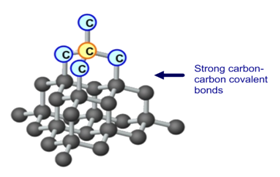
Diamond has a high melting point because it is a giant covalent structure with many strong covalent bonds that require a lot of energy to break.
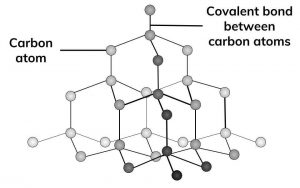
Allotropes are different forms of the same element. Three different allotropes of carbon are shown here as examples: diamond, graphite and C60 fullerene.
Diamond is made up of only carbon atoms, in a giant 3D lattice, where each of those atoms has a strong covalent bonds to 4 other carbon. Every one of carbon’s 4 outer electrons is involved in one of these strong covalent bonds.
Diamond is extremely hard because it is a giant covalent structure with many strong covalent bonds.
Because it is hard, diamond is used in high speed cutting tools, eg diamond-tipped saws.
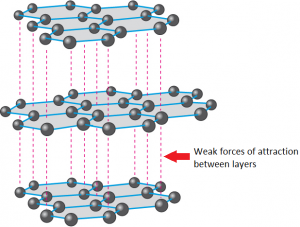
Graphite is also made of only carbon atoms, and is also a giant structure, but it is formed of layers where each carbon atom has a strong covalent bond to 3 other carbons. This means each carbon atom has one electron not involved in a covalent bond, and these electrons form a sea of delocalised electrons between the layers.
Even though it is a non-metal, graphite can conduct electricity because the delocalised electrons are free to move.
Each layer is a giant structure, with weak forces of attraction between the layers. These layers can easily slide over each other.
Graphite is soft and slippery because it has weak forces of attraction between layers. It is used as a lubricant and in pencils because it is soft and slippery.
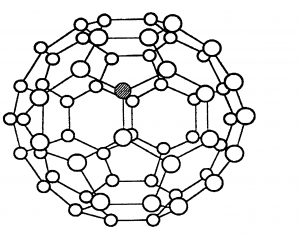
C60 fullerene which is a simple molecular structure (also known as a buckyball) is also made of only carbon atoms, but it forms molecules of 60 carbon atoms. The molecule has weak intermolecular forces of attraction between them which take little energy to overcome. Hence C60 fullerene has a low melting point, and it is soft.
C60 fullerene cannot conduct electricity. Although in each molecule every carbon is only covalently bonded to 3 others and the other electrons are delocalised, these electrons cannot jump between different molecules.
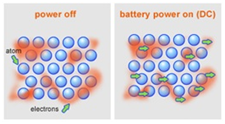
Electric current is a flow of charged particles that can move.
Covalent compounds do not conduct electricity.
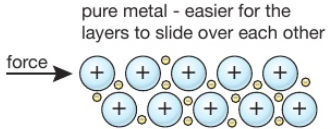
When metal atoms join together the outer electrons become ‘delocalised’ which means they are free to move throughout the whole structure.
Metals have a giant regular arrangement of layers of positive ions surrounded by a sea of delocalised electrons.
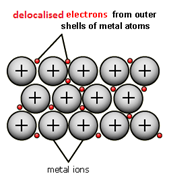
Metallic bonding is the strong electrostatic attraction between positive metal ions and a sea of delocalised electrons.

Metals are good conductors because they have delocalised electrons which are free to move.
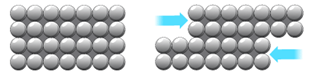
Metals are malleable (can be hammered into shape) because they have layers of ions that can slide over each other.
This video explains the structure of alloys, including different forms of steel.
Note that a slight improvement could be made in the explanation: the structure of metals should be described as “layers of metal IONS in a sea of delocalised electrons”.
Electrical conductivity is the movement of charged particles.
In this case, charged particles means either delocalised electrons or ions.
These particles need to be free to move in a substance for that substance to be conductive.
Covalent compounds do not conduct electricity because there are no charged particles that are free to move.
Ionic compounds only conduct electricity only when molten or in solution.
When solid the ions are not free to move.
When molten or in solution the ions are free to move.
| Aluminium | |
|---|---|
| Use | Property |
| Aircrafts and cans | Low density / resists corrosion |
| Power cables | Conducts electricity / ductile |
| Pots and pans | Low density / strong (when alloyed) / good conductor of electricity and heat |
Aluminium resists corrosion because it has a very thin, but very strong, layer of aluminium oxide on the surface.
| Copper | |
|---|---|
| Use | Property |
| Electrical wires | very good conductor of electricity and ductile |
| Pots and pans | very good conductor of heat / very unreactive / malleable |
| Water pipes | unreactive / malleable |
| Surfaces in hospitals | antimicrobial properties / malleable |
| Iron | |
|---|---|
| Use | Property |
| Buildings | Strong |
| Saucepans | Conducts heat / high melting point / malleable |
| Steel | ||
|---|---|---|
| Type of steel | Iron mixed with | Some uses |
| Mild steel | up to 0.25% carbon | nails, car bodies, ship building, girders |
| High-carbon steel | 0.6%-1.2% carbon | cutting tools, masonry nails |
| Stainless steel | Chromium (and nickel) | cutlery, cooking utensils, kitchen sinks |
Mild steel is a strong material that can easily be hammered into various shapes (malleable). It rusts easily.
High-carbon steel is harder than mild steel but more brittle (not as malleable).
Stainless steel forms a strong, protective oxide layer so is very resistant to corrosion.
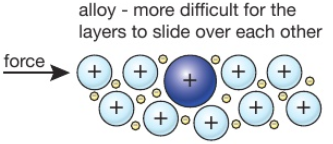
An alloy is a mixture of a metal with, usually, other metals or carbon.
For example, brass is a alloy of copper and zinc, and steel is an alloy of iron and carbon.
Alloys are harder than the individual pure metals from which they are made.
In an alloy, the different elements have slightly different sized atoms. This breaks up the regular lattice arrangement and makes it more difficult for layers of ions to slide over each other.