Ions quiz
Electrons are found in a series of shells (or energy levels) around the nucleus of an atom.
Each energy level can only hold a certain number of electrons. Low energy levels are always filled up first.
Rules for working out the arrangement (configuration) of electrons:
Example – chlorine (Cl)
1) Use the periodic table to look up the atomic number. Chlorine’s atomic number (number of protons) is 17.
2) Remember the number of protons = number of electrons. Therefore chlorine has 17 electrons.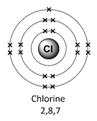
3) Arrange the electrons in levels (shells):
Therefore the electron arrangement for chlorine (17 electrons in total) will be written as 2,8,7
4) Check to make sure that the electrons add up to the right number
The electron arrangement can also be draw in a diagram.
Electron arrangement for the first 20 elements:
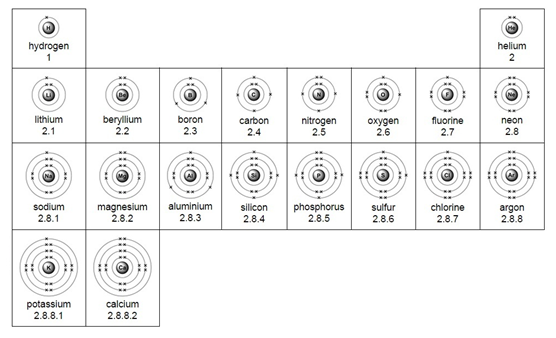
Elements in the same group have the same number of electrons in their outer shell.
This is why elements from the same group have similar properties.
Elements in the same group of the periodic table have the same number of electrons in their outer shells, which means they have similar chemical properties.
The noble gases are inert (unreactive) because they have a full outer shell of electrons.
Example:
Sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
Word equation:
sodium + water –> sodium hydroxide + hydrogen
Writing the chemical equation
A chemical equation represents what happens in terms of atoms in a chemical reaction.
Step 1: To write a chemical equation we need to know the chemical formulae of the substances.
Na + H2O –> NaOH + H2
Step 2: The next step is to balance the equation: write a large number before each compound so the number of atoms of each element on the left hand side (reactants) matches the number on the right (products). This large number is the amount of each compound or element.
During this balancing stage the actual formulas for each compound must not be changed. Only the number of each compound changes.
2Na + 2H2O –> 2NaOH + H2
If asked for an equation, the chemical equation must be given.
State symbols are used to show what physical state the reactants and products are in.
| State symbols | Physical state |
|---|---|
| (s) | Solid |
| (l) | Liquid |
| (g) | Gas |
| (aq) | Aqueous solution (dissolved in water) |
Example:
A solid piece of sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
2Na(s) + 2H2O(l) –> 2NaOH(aq) + H2(g)
Ions are electrically charged particles formed when atoms lose or gain electrons.
They have the same electronic structures as noble gases.
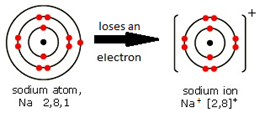
Metal atoms form positive ions (cations).
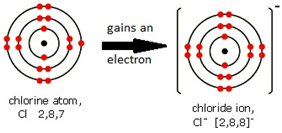
Non-metal atoms form negative ions (anions).
Knowing the answer to “What’s an ion?” is absolutely key to Chemistry.
If you aren’t sure what an ion is, or why the idea of ions is crucial to Chemistry, then you should watch this video:
This should give you a ruff idea of what the difference is between ionic and covalent bonding.
In an electrically neutral ATOM, the number of electrons equals the number of protons.
However, an ION is an atom (or group of atoms) which has either gained or lost some electrons, so for an ION the number of electrons does not equal the number of protons.
This excellent Tyler de Witt video explains this clearly:
When given this information of the following ions, it is possible to work out the formulae of ionic compounds which include these ions.
| Name of Ion | Formula | Charge |
|---|---|---|
| Sulfate | SO42- | -2 |
| Carbonate | CO32- | -2 |
| Nitrate | NO3- | -1 |
| Hydroxide | OH- | -1 |
| Ammonium | NH4+ | +1 |
| Silver ion | Ag+ | +1 |
| Zinc ion | Zn2+ | +2 |
| Hydrogen ion | H+ | +1 |
| Copper (II) ion | Cu2+ | +2 |
| Iron (II) ion | Fe2+ | +2 |
| Iron (III) ion | Fe3+ | +3 |
| Lead (II) ion | Pb2+ | +2 |
Ion charges on the periodic table
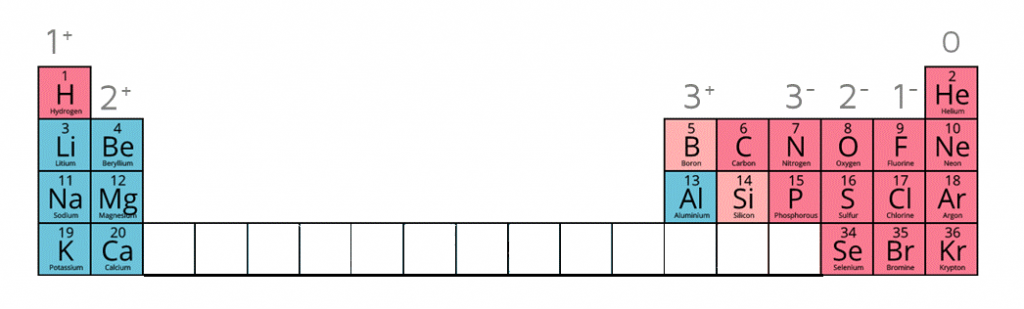
Writing the electron configuration of an atom allows you to work out the electron configuration of the ion and therefore the charge on the ion.
Examples:
Atom = Mg
Electron configuration = 2,8,2
remove the two electrons from the outer shell to achieve the same electron configuration as the nearest noble gas, Neon (Ne 2,8)
Ion = Mg2+
Atom = O
Electron configuration = 2,6
add two electrons to the outer shell to achieve the same electron configuration as the nearest noble gas, Neon (Ne 2,8)
Ion = O2- [2,8]2-
This excellent video from the lovely Tyler de Witt explains how to write the formula of an ionic compound:
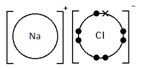
Sodium chloride, NaCl
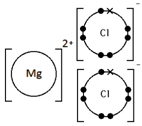
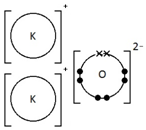
Calcium oxide, CaO
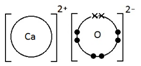
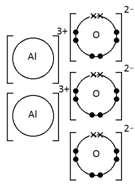
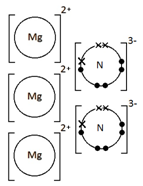
Ionic bonding: a strong electrostatic attraction between oppositely charged ions.
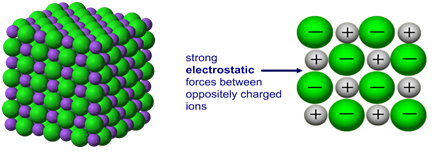
Ionic compounds have high melting and boiling points because they have a giant structure with strong electrostatic forces between oppositely charged ions that require a lot of energy to break.
Giant 3D lattice of sodium chloride (NaCl)
These videos are a really good introduction to ionic bonding: