3.01 use the following units: degree (°), hertz (Hz), metre (m), metre/second (m/s) and second (s)
the units for:
angle = degree (°)
frequency = hertz (Hz)
wavelength = metre (m)
velocity = metre/second (m/s)
time = second (s)
the units for:
angle = degree (°)
frequency = hertz (Hz)
wavelength = metre (m)
velocity = metre/second (m/s)
time = second (s)
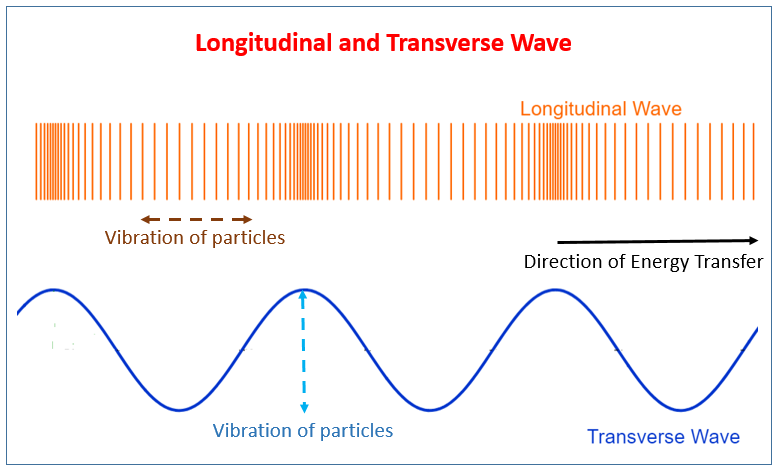
Transverse Waves:
Longitudinal Waves:
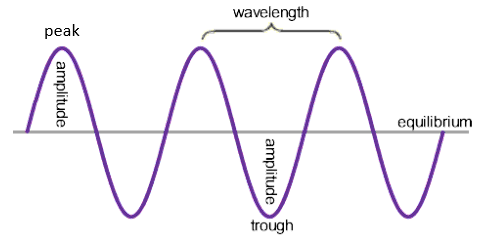
Key Definitions:
Waves can transfer energy and information with out transferring matter, for example sun light, it transfers energy as it makes the earth warm without bringing any matter.
wave speed (m/s) = frequency (Hz) x Wavelength (m)
Frequency (Hz) = 1/ Time Period (s)
You will need to use any of the in 3.05 and 3.06 to solve problems to do with sound waves and electromagnetic (light) waves