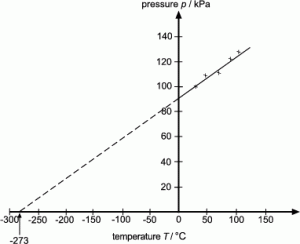
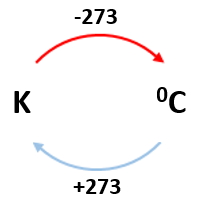
5.01 use the following units: degree Celsius (°C), Kelvin (K), joule (J), kilogram (kg), kilogram/metre3 (kg/m3), metre (m), metre2 (m2), metre3 (m3), metre/second (m/s), metre/second2 (m/s2), newton (N) and pascal (Pa)
The units for:
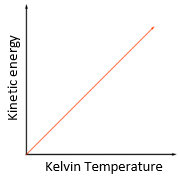
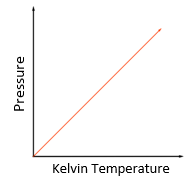
temperature: degree Celsius (°C) or Kelvin (K)
Energy: Joule (J)
mass: Kilogram (kg)
density: kilogram/metre cubed (kg/m3)
distance: metre (m)
area: metre squared (m2)
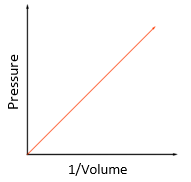
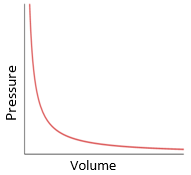
volume: metre cubed (m3)
velocity: metre per second (m/s)
acceleration: metre per second squared (m/s2)
force: newton (N)
pressure: pascal (Pa)