8.02 know that: the universe is a large collection of billions of galaxies, a galaxy is a large collection of billions of stars, our solar system is in the Milky Way galaxy
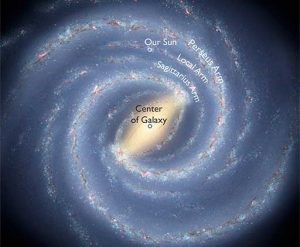

The Milky Way galaxy contains billions of stars
The Universe – billions of galaxies
