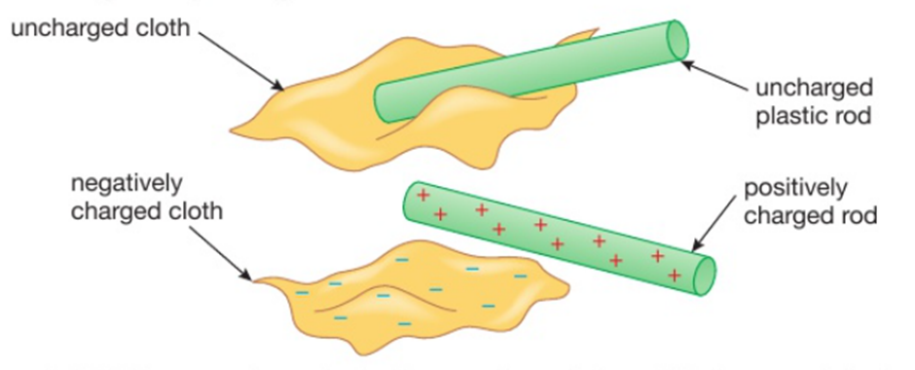
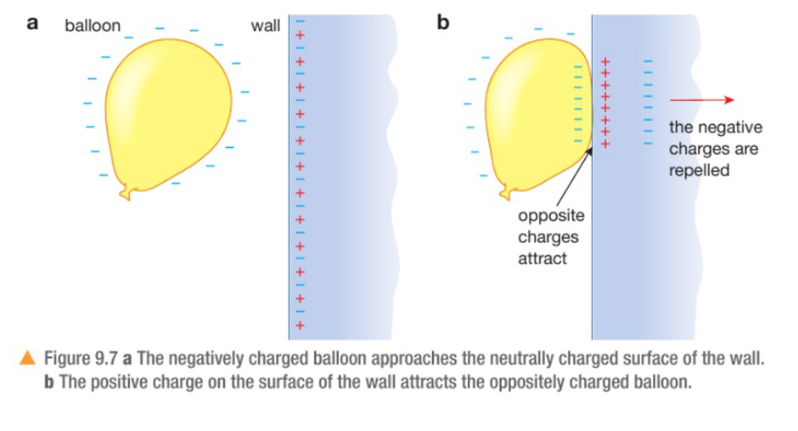
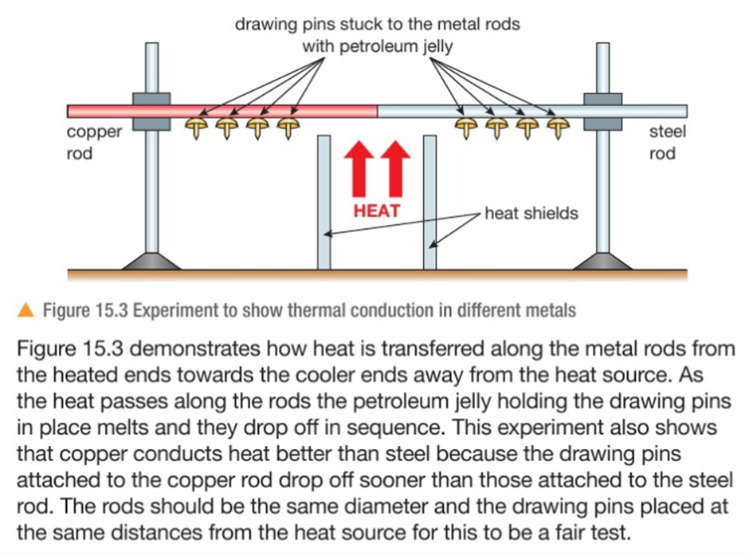
Conduction is the transfer of thermal energy through a substance by the vibration of the atoms within the substance. Metals are good conductors because they have free electrons that can move easily through the metal, making the transfer of energy happen faster.
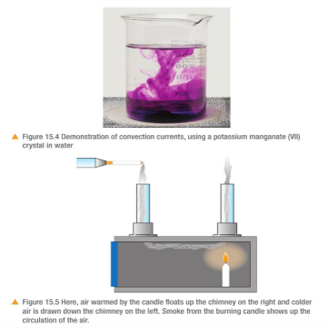
Convection occurs in a liquid or gas. These expand when heated because the particles move faster and take up more volume – the particles remain the same size but become further apart. The hot liquid or gas is less dense, so it rises into colder areas. The denser, colder liquid or gas falls into the warm areas. In this way, convection currents are set up which transfer heat from place to place.
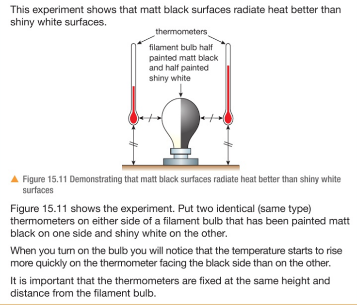
Thermal radiation is the transfer of energy by infrared (IR) waves. These travel very quickly in straight lines.