3.01 use the following units: degree (°), hertz (Hz), metre (m), metre/second (m/s) and second (s)
the units for:
angle = degree (°)
frequency = hertz (Hz)
wavelength = metre (m)
velocity = metre/second (m/s)
time = second (s)
the units for:
angle = degree (°)
frequency = hertz (Hz)
wavelength = metre (m)
velocity = metre/second (m/s)
time = second (s)
Transverse Waves:
Longitudinal Waves:
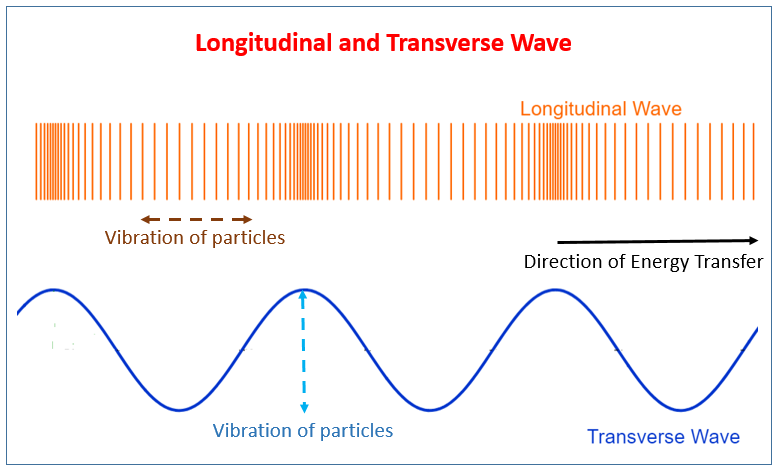
Key Definitions:
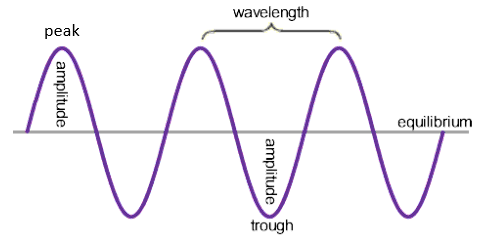
wave speed (m/s) = frequency (Hz) x Wavelength (m)
Electromagnetic Spectrum:
Radio Waves
Microwaves
Infrared (IR)
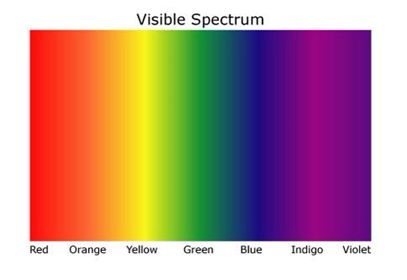
Visible Light
Ultraviolet (UV)
X – Rays
Gamma Rays
these are written in order of increasing frequency, lowest at the top
and decreasing wavelength, lowest at the bottom.
the colours displayed are in order of lowest frequency to the left highest frequency to the right.
|
uses of electromagnetic radiations, including: |
the detrimental effects of excessive exposure of the human body to electromagnetic waves:
• microwaves: internal heating of body tissue
• infrared: skin burns
• ultraviolet: damage to surface cells and blindness
• gamma rays: cancer, mutation
to reduce the risks: