7.17 know that nuclear reactions, including fission, fusion and radioactive decay, can be a source of energy
- Nuclear Fission:
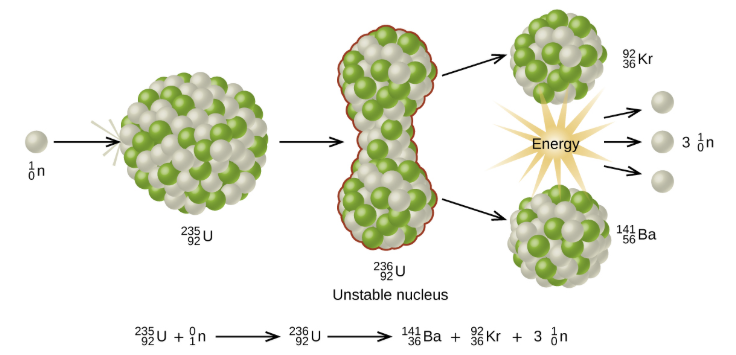
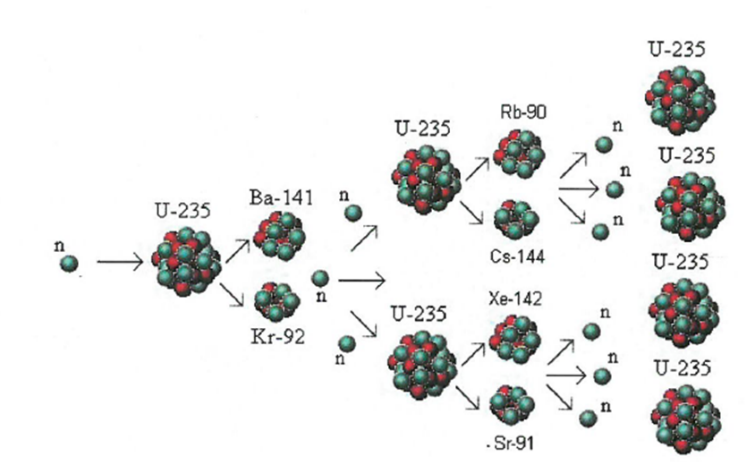
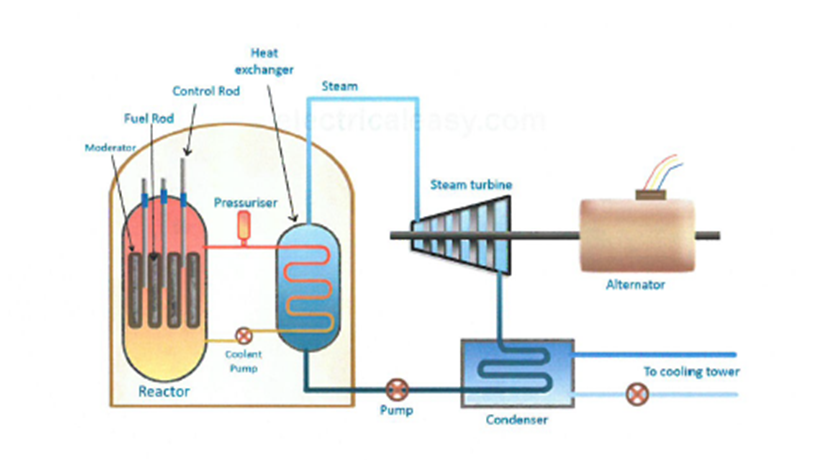
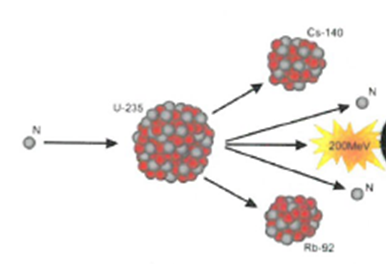
The process where heavy atoms are split into smaller, lighter atoms. This releases energy.
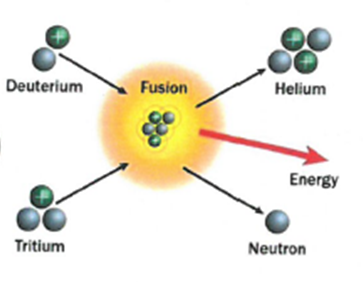
- Nuclear Fission:
The process where lighter atoms are forced to join together to make heavier atoms. This releases energy.
- Radioactive Decay:
Within the core of the Earth, radioactive isotopes of elements such as uranium, thorium and potassium provide a large proportion of the heat within the Earth through radioactive decay.