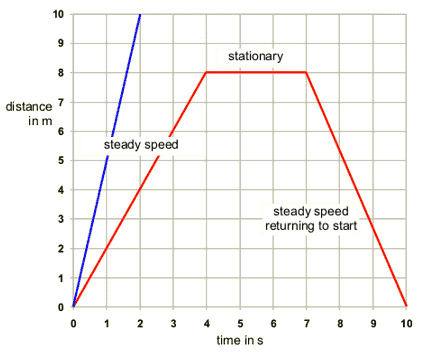
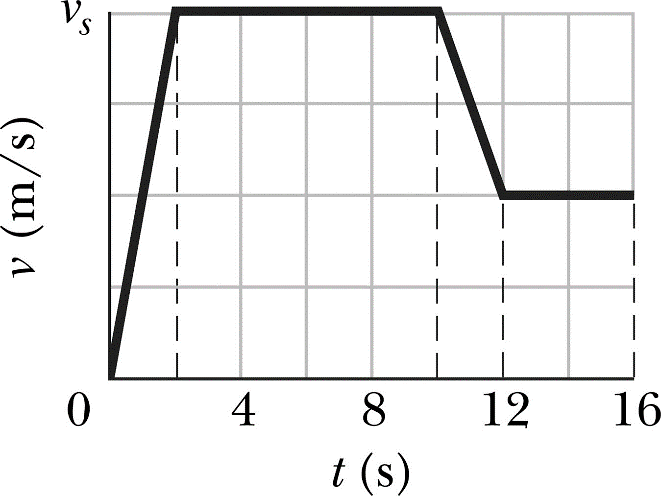
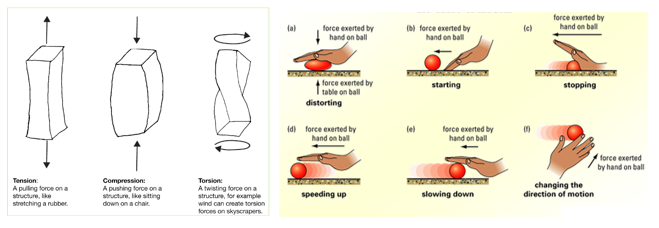
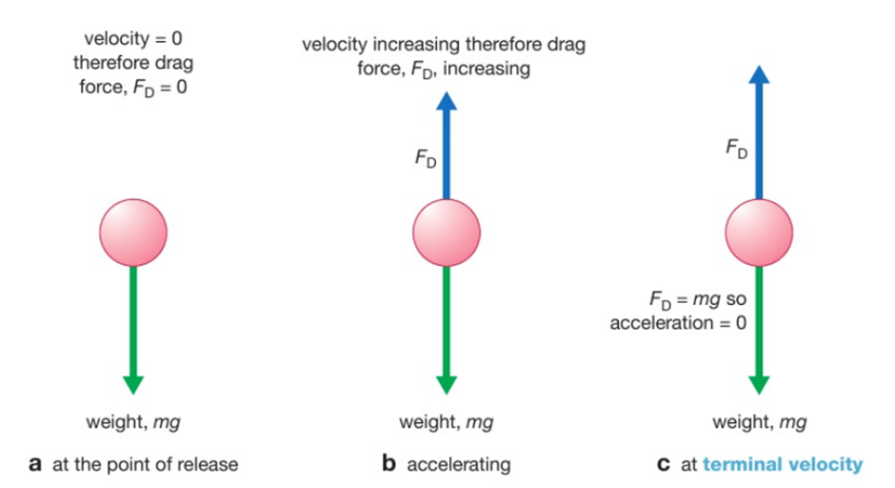
Increasing the surface area of a solid:
- more particles exposed
- more frequent collisions
- increase the rate of a reaction
Increasing the concentration of a solution or pressure of a gas:
- more particles in same space
- more frequent collisions
- increase rate of reaction
Increasing the temperature:
- particles have more kinetic energy
- more frequent collisions
- and a higher proportion of those collisions are successful because the collision energy is greater or equal to the activation energy
- increase rate of reaction