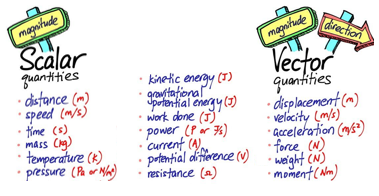
In a substitution reaction an atom or group of atoms is replaced by a different atom or group of atoms. For example when ethane reacts with bromine gas one of the hydrogen atoms in ethane is substituted by one of the atoms of bromine from within the bromine molecule:
CH₃-CH₃ + Br-Br → CH₃-CH₂Br + H-Br
ethane + bromine → bromoethane + hydrogen bromide
An addition reaction occurs when an atom or group of atoms is added to a molecule without taking anything away. For example when ethene reacts with bromine gas, the product is simply the addition of the two molecules:
CH₂=CH₂ + Br-Br → CH₂Br-CH₂Br
A combustion reaction is another way to say ‘burning’ and is a reaction with oxygen. Combustion of hydrocarbons with excess oxygen gives the products water and carbon dioxide, and also releases heat energy (exothermic reaction). Two examples the combustion of propane and the combustion of butene:
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
C₄H₈ + 6O₂ → 4CO₂ + 4H₂O