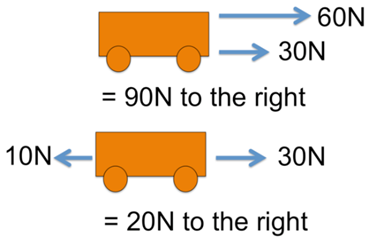
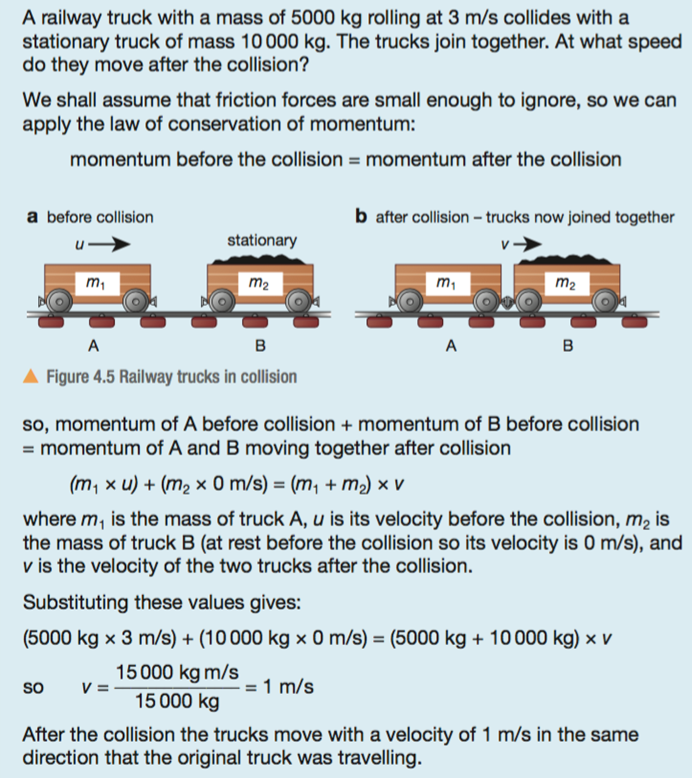
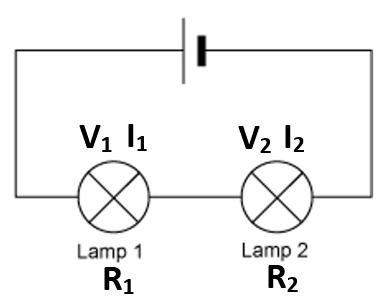
Yield is how much product you get from a chemical reaction.
The theoretical yield is the amount of product that you would expect to get. This is calculated using reacting mass calculations.
In most chemical reactions, however, you rarely achieved your theoretical yield.
For example, in the following reaction:
CaCO3 –> CaO + CO2
You might expect to achieve a theoretical yield of 56 g of CaO from 100 g of CaCO3.
However, what if the actual yield is only 48 g of CaO.
By using the following formula, the % yield can be calculated: