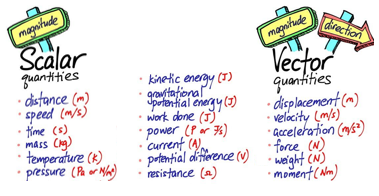
Pure substances, such as an element or a compound, melt and boil at fixed temperatures.
However, mixtures melt and boil over a range of temperatures.
Example: although pure water boils at 100⁰C, the addition of 10g of sodium chloride (NaCl) to 1000cm³ of water will raise the boiling point to 100.2⁰C.
Example: although pure water melts at 0⁰C, the addition of 10g of sodium chloride (NaCl) to 1000cm³ of water will lower the melting point to -0.6⁰C.