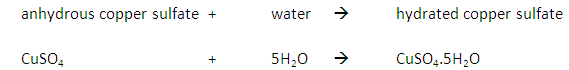Simple Molecules & Covalent Bonding quiz
Diffusion is the spreading out of particles in a gas or liquid. There is a net movement of particles from areas of high concentration to areas of low concentration until a uniform concentration is achieved.
i) dilution of coloured solutions
 Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
Dissolving potassium manganate(VII) in water demonstrates that the diffusion in liquids is very slow because there are only small gaps between the liquid particles into which other particles diffuse.
The random motion of particles cause the purple colour to eventually be evenly spread out throughout the water.
Adding more water to the solution causes the potassium manganate(VII) particles to spread out further apart therefore the solutions becomes less purple. This is called dilution.
ii) diffusion experiments
When ammonia gas and hydrogen chloride gas mix, they react together to form a white solid called ammonium chloride.
ammonia + hydrogen chloride –> ammonium chloride
NH3(g) + HCl(g) –> NH4Cl(s)
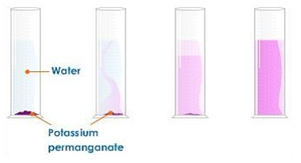
A cotton wool pad was soaked in ammonia solution and another was soaked in hydrogen chloride solution. The two pads were then put into opposite ends of a dry glass tube at the same time.
The white ring of ammonium chloride forms closer to the hydrochloric acid end because ammonia particles are lighter than hydrogen chloride particles and therefore travel faster.
Even though these particles travel at several hundred metres per second, it takes about 5 min for the ring to form. This is because the particles move in random directions and will collide with air particles in the tube.
When a solid dissolves in a liquid:
Solubility is defined in terms of the maximum mass of a solute that dissolves in 100g of solvent. The mass depends on the temperature.
For example, the solubility of sodium chloride (NaCl) in water at 25⁰C is about 36g per 100g of water.
The solubility of solids changes as temperature changes. This can be plotted on a solubility curve.
The salts shown on this graph are typical: the solubility increases as temperature increases.
For example, the graph above shows that in 100g of water at 50⁰C the maximum mass of potassium nitrate (KNO₃) which will dissolve is 80g.
However, if the temperature were 80⁰C a mass of 160g of potassium nitrate (KNO₃) would dissolve in 100g of water.
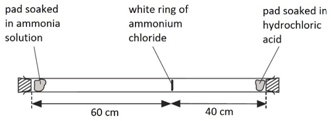
At a chosen temperature (e.g. 40⁰C) a saturated solution is created of potassium nitrate (KNO₃) for example.
Some of this solution (not any residual solid) is poured off and weighed. The water is then evaporated from this solution to leave a residue of potassium nitrate which is then weighed.
The difference between the two measured masses is the mass of evaporated water.
The solubility, in grams per 100g of water, is equal to 100 times the mass of potassium nitrate residue divided by the mass of evaporated water.
Paper chromatography can be used to investigate the composition of a mixture.
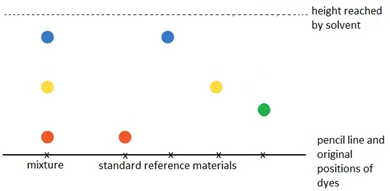
A baseline is drawn on the paper. The mixture is spotted onto the baseline alongside known or standard reference materials. The end of the paper is then put into a solvent which runs up the paper and through the spots, taking some or all of the dyes with it.
Different dyes will travel different heights up the paper.
The resulting pattern of dyes is called a chromatogram.
In the example shown, the mixture is shown to contain the red, blue and yellow dyes. This can be seen because these dots which resulted from the mixture have travelled the same distance up the paper as have the red, blue and yellow standard reference materials.
When analysing a chromatogram, the mixture being analysed is compared to standard reference materials by measuring how far the various dyes have travelled up the paper from the baseline where they started.
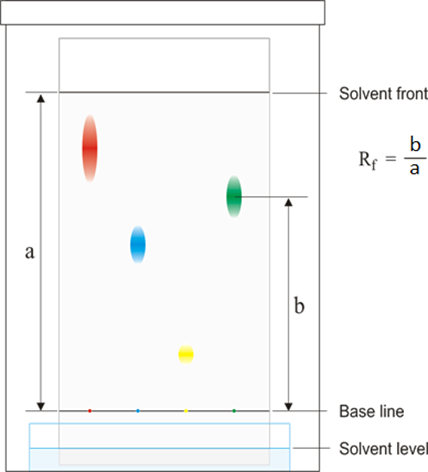
For each dye, the Rf value is calculated. To do this, 2 distances are measured:
The Rf value is calculated as follows:
If the Rf value of one of the components of the mixture equals the Rf value of one of the standard reference materials then that component is know to be that reference material.
Note that because the solvent always travels at least as far as the highest dye, the Rf value is always between 0 and 1.
Dyes which are more soluble will have higher Rf values than less soluble dyes. In other words, more soluble dyes move further up the paper. The extreme case of this is for insoluble dyes which don’t move at all (Rf value = 0). The other aspect affecting how far a dye travels is the affinity that dye has for the paper (how well it ‘sticks’ to the paper).
Common solvents are water or ethanol. The choice of solvent depends on whether most of the dyes are soluble in that solvent.
Atom: An atom is the smallest part of an element.
Molecule: A molecule is made of a fixed number of atoms covalently bonded together.
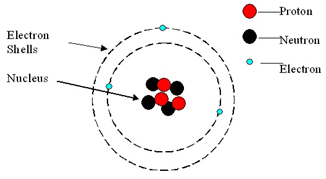
An atom consists of a central nucleus, composed of protons and neutrons.
This is surrounded by electrons, orbiting in shells (energy levels).
Atoms are neutral because the numbers of electrons and protons are equal.
| Mass | Charge | |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | negligible (1/1836) | -1 |
Atomic number: The number of protons in an atom.
Mass number: The number of protons and neutrons in an atom.
Relative atomic mass (Ar): The average mass of an atom compared to 1/12th the mass of carbon-12.
The elements in the Periodic Table are arranged in order of increasing atomic number.
Columns are called Groups. They indicate the number of electrons in the outer shell of an atom.
Rows are called Periods. They indicate the number of shells (energy levels) in an atom.
Electrons are found in a series of shells (or energy levels) around the nucleus of an atom.
Each energy level can only hold a certain number of electrons. Low energy levels are always filled up first.
Rules for working out the arrangement (configuration) of electrons:
Example – chlorine (Cl)
1) Use the periodic table to look up the atomic number. Chlorine’s atomic number (number of protons) is 17.
2) Remember the number of protons = number of electrons. Therefore chlorine has 17 electrons.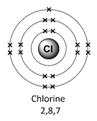
3) Arrange the electrons in levels (shells):
Therefore the electron arrangement for chlorine (17 electrons in total) will be written as 2,8,7
4) Check to make sure that the electrons add up to the right number
The electron arrangement can also be draw in a diagram.
Electron arrangement for the first 20 elements:
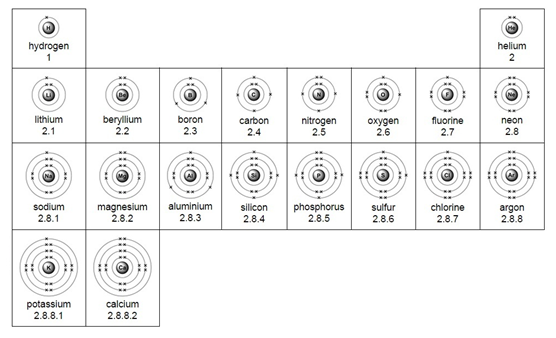
Metals on the left of the Periodic Table.
Non-Metals on the top-right, plus Hydrogen.
Elements in the same group have the same number of electrons in their outer shell.
This is why elements from the same group have similar properties.
Elements in the same group of the periodic table have the same number of electrons in their outer shells, which means they have similar chemical properties.
The noble gases are inert (unreactive) because they have a full outer shell of electrons.
This should give you a ruff idea of what the difference is between ionic and covalent bonding.
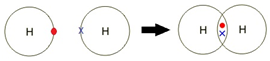
A covalent bond is formed between two non-metal atoms by sharing a pair of electrons in order to fill the outer shell.
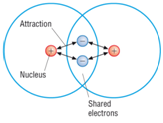
Covalent bonding: a strong attraction between a shared pair of electrons and two nuclei.
These videos are a good introduction to covalent bonding:
This excellent Tyler de Witt video addresses a common mistake around diatomic elements (H2, N2, O2, F2, Cl2, Br2, I2).
For example, if chlorine is in its elemental form (i.e. not bonded in a compound) the formula is Cl2, but when chlorine is bonded to sodium the formula of sodium chloride is NaCl.
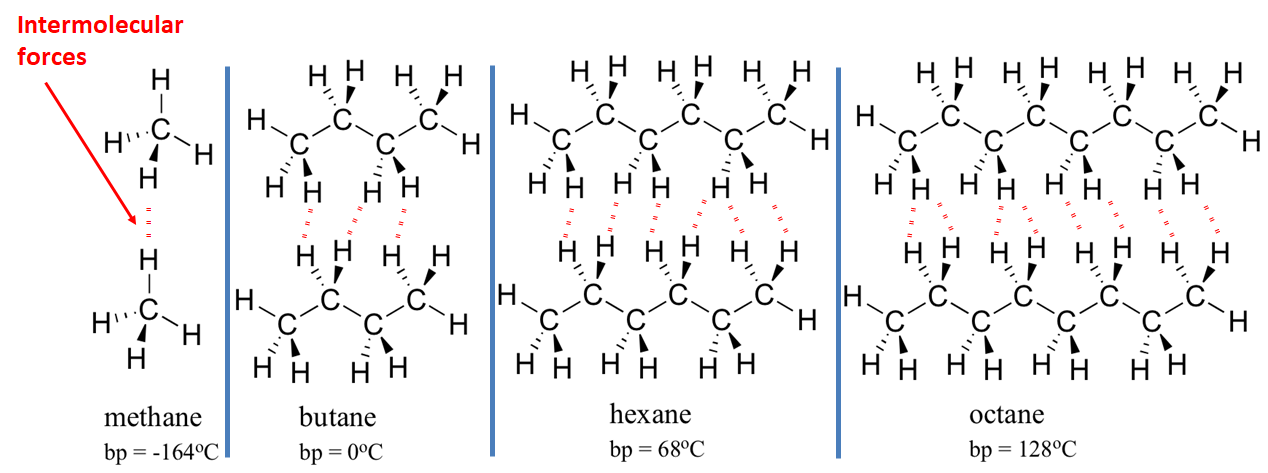
Carbon dioxide (CO2) has a simple molecular structure. This just means that it is made up of molecules.
Within each molecule are atoms bonded to each other covalently. These covalent bonds inside the molecules are strong.
However, between the molecules are weak forces of attraction that require little energy to break. These forces are not covalent bonds. This is why simple molecular substances such as carbon dioxide have a low boiling point.
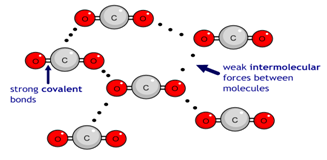
So when carbon dioxide changes from a solid to a gas, for example, that process can be represented as:
CO₂ (s) → CO₂ (g)
Notice that even though there has been a dramatic change of state from solid to gas, the substance before and after the change is always made up of carbon dioxide molecules. During the change of the state the covalent bonds within each molecule remain unbroken. Instead it is the weak forces of attraction between the molecules which have been overcome.
Larger molecules tend to have higher boiling points.
This is because larger molecules (molecules with more mass) have more forces of attraction between them. These forces, although weak, must be overcome if the substance is to boil, and larger molecules have more attractions which must be overcome.
Air is a mixture of different gases.
The abundance of gases in the air is as follows:
| Gas | % by volume |
|---|---|
| Nitrogen, N2 | 78.1 |
| Oxygen, O2 | 21.0 |
| Argon, Ar | 0.9 |
| Carbon dioxide, CO2 | 0.04 |
The following 3 experiments can be used to determine that oxygen (O2) makes up approximately 20% by volume of the composition of air.
Copper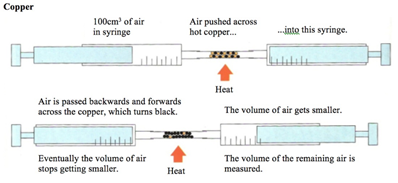
The copper is in excess and uses up the oxygen to form copper oxide (CuO).
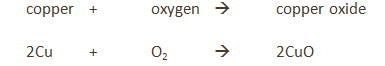
All the oxygen in the air is therefore used up, and so the volume of the air decreases by about 20% (the percentage of oxygen in air).
Iron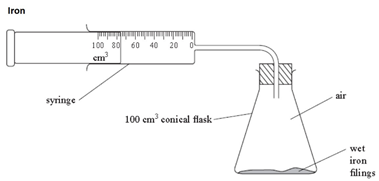
The iron reacts with the oxygen in the air (rusting).
As long as the iron and water are in excess, the total volume of air enclosed by the apparatus decreases by about a fifth (20%) over several days.
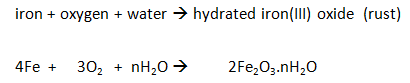
Phosphorus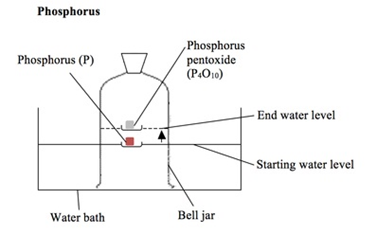
The phosphorus is lit with a hot wire.
It reacts with the oxygen in the air and causes the water level in the bell jar to rise by about 20%.
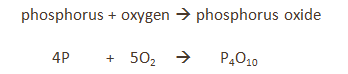
The following 3 experiments can be used to determine that oxygen (O2) makes up approximately 20% by volume of air.
Copper
The copper is in excess and uses up the oxygen to form copper oxide (CuO).
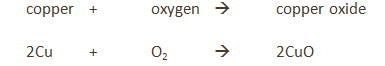
All the oxygen in the air is therefore used up, and so the volume of the air decreases by about 20% (the percentage of oxygen in air).
Iron
The iron reacts with the oxygen in the air (rusting).
As long as the iron, oxygen and water are all in excess, the total volume of air enclosed by the apparatus decreases by about a fifth (20%) over several days.
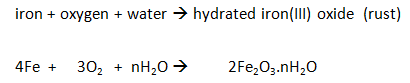
Phosphorus
The phosphorus is lit with a hot wire.
It reacts with the oxygen in the air and causes the water level in the bell jar to rise by about 20%.
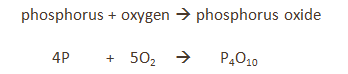
Tests for gases
| Gas | Test | Result if gas present |
|---|---|---|
| hydrogen (H2) | Use a lit splint | Gas pops |
| oxygen (O2) | Use a glowing splint | Glowing splint relights |
| carbon dioxide (CO2) | Bubble the gas through limewater | Limewater turns cloudy |
| ammonia (NH3) | Use red litmus paper | Turns damp red litmus paper blue |
| chlorine (Cl2) | Use damp litmus paper | Turns damp litmus paper white (bleaches) |
Add anhydrous copper (II) sulfate (CuSO4) to a sample.
If water is present the anhydrous copper (II) sulfate will change from white to blue.