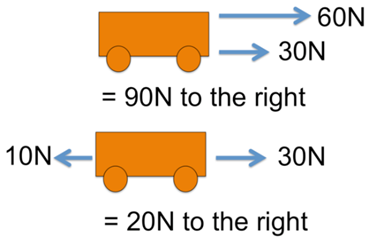
75% of chlorine atoms are the type 35Cl (have a mass number of 35)
25% of chlorine atoms are of the type 37Cl (have a mass number of 37)
In order to calculate the relative atomic mass (Ar) of chlorine, the following steps are used:
- Multiply the mass of each isotope by its relative abundance
- Add those together
- Divide by the sum of the relative abundances (normally 100)
Example question:
A sample of bromine contained the two isotopes in the following proportions: bromine-79 = 50.7% and bromine-81 = 49.3%.
Calculate the relative atomic mass (Ar) of bromine.