Condensation Polymers quiz
When fuels are burned in vehicle engines, high temperatures are reached.
At these high temperatures nitrogen and oxygen from the air react to produce nitrogen oxides:
nitrogen + oxygen → nitrogen oxides
eg
N2 (g) + O2 (g) → 2NO (g)
In the atmosphere these nitrogen oxides can combine with water to produce nitric acid (HNO3).
Fossil fuels such as coal, gas and oil are derived from crude oil.
These fuels are hydrocarbons, but also include impurities such as sulfur.
When the fuels are burned, sulfur dioxide is produced which can escape into the atmosphere:
S (s) + O₂ (g) → SO₂ (g)
Acids formed in the atmosphere can fall as acid rain. This can be a major problem, killing trees and fish in lakes. The acid rain also corrodes limestone buildings and marble statues since these are both made of calcium carbonate (CaCO₃). Some metals such as iron are also attacked by acid rain.
Sulfur dioxide released into the atmosphere from the burning of fossil fuels can react with water and oxygen to make sulfuric acid (H₂SO₄):
2SO₂ (g) + 2H₂O (l) + O₂ (g) → 2H₂SO₄ (aq)
Also, if sulfur dioxide in the atmosphere reacts with just water, a weaker acid called sulfurous acid (H₂SO₃) is formed:
SO₂ (g) + H₂O (l) → H₂SO₃ (aq)
In car engines the temperature is high enough for the nitrogen in the air to react with oxygen to produce oxides of nitrogen, e.g:
N₂ (g) + O₂ (g) → NO₂ (g)
In the atmosphere these nitrogen oxides can produce nitric acid (HNO₃).
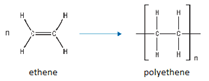
Monomers join together to form a long chain.
Polymer contains only single bonds.
To deduce the structure of the monomer from a repeat unit:
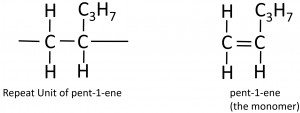
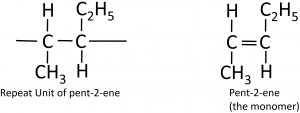
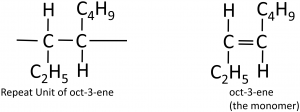
Here’s a more complicated example, going from the polymer to the structure of the monomer
This video introduces addition polymers:
Polymers are inert (unreactive) as they have strong C-C bonds.
This makes them non-biodegradeable.
Biodegradable: the breakdown of a substance by microorganisms.
if burnt the addition polymers could produce toxic gases such as carbon monoxide and hydrogen chloride.
Condensation polymers are formed by a condensation reaction.
These polymers are formed by the combination of two different monomers, such as a dicarboxylic acid and a diol.
When these particular monomers join in an alternating pattern they form a long polymer called a polyester. Where each monomer joins to the next, a separate molecule of water is also produced.
Polyesters are polymers formed when two types of monomer join together alternately. Where each joins to the next a small molecule, such as water or hydrogen chloride, is lost. This is called a condensation polymerisation reaction.
One of the monomers is a diol, an alcohol with a -OH functional group at each end. An example is hexane-1,6-diol which has the structural formula CH₂OHCH₂CH₂CH₂CH₂CH₂OH and the displayed formula:
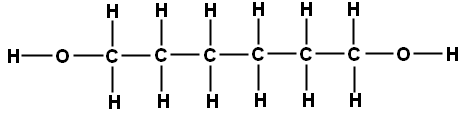
Since it is only the -OH functional groups which are important for polymerisation, this can we re-written with the central block of carbons represented as a block:
![]()
The other monomer is a dicarboxylic acid, a molecule with a -COOH functional group at each end. An example is hexane-1,6-dioic acid which has the structural formula HOOCCH₂CH₂CH₂CH₂COOH and the displayed formula:
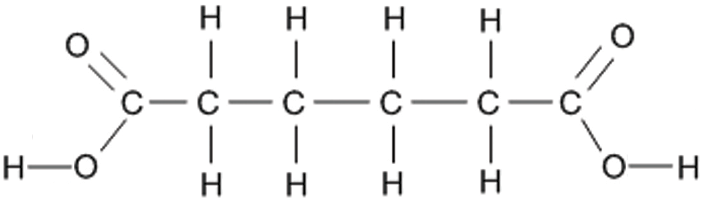
Since it is only the -COOH functional groups which are important for polymerisation, this can we re-written with the central block of 4 carbons represented as a block:
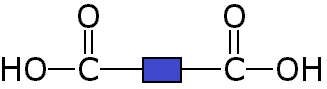
These two different types of monomer (the diol and the dicarboxylic acid) can join to form a polymer with the loss of a water molecule at every bond. As above, this can be simplified by only looking at the functional groups and representing the other carbons as blocks, so the whole process looks like:
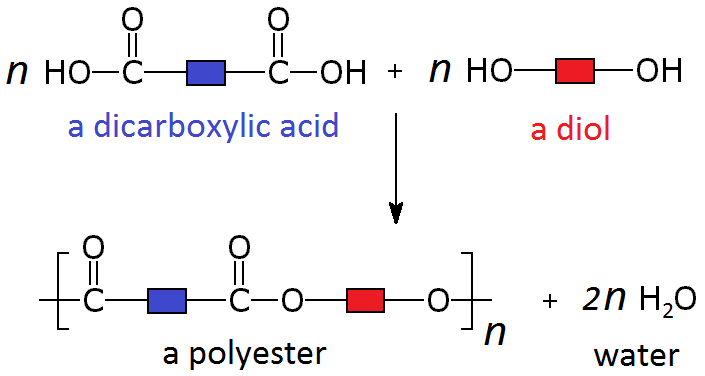
A simple example of this is the condensation polymerisation reaction between ethanedioic acid and ethandiol:
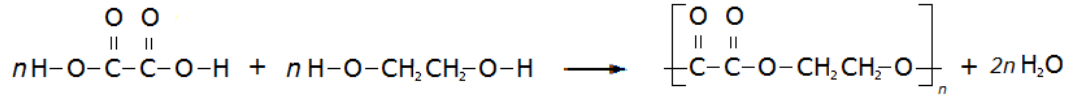
This video introduces the idea of condensation polymers:
There are environmental issues with the disposal of condensation polymers, though because of their ester linkage the issues are not as severe as with addition polymers. Normally condensation polymers can take hundreds of years to break down, but chemists has developed biopolyesters which break down much more quickly.