4:07 know that crude oil is a mixture of hydrocarbons
Crude Oil is a mixture of hydrocarbons.
Crude Oil is a mixture of hydrocarbons.
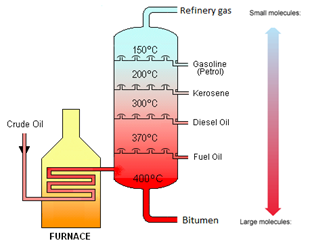
Crude oil is separated into fractions by the process of fractional distillation.
| Fraction | Use |
|---|---|
| Refinery gases | Bottled gas |
| Gasoline | Fuel for cars |
| Kerosene | Fuel for aeroplanes |
| Diesel Oil | Fuel for lorries |
| Fuel Oil | Fuel for ships |
| Bitumen | Road Surfacing |
The boiling point increases as the number of carbon atoms (chain length) increases.
The viscosity increases as the number of carbon atoms (chain length) increases.
The greater the number of carbon atoms (chain length), the darker in colour that fraction is.
The viscosity of a fluid describes how easily it flows. Water has a low viscosity, it flows very easily. Crude oil has a higher viscosity than water, it does not flow very easily.
| Fractions (in order) | Properties |
|---|---|
| Refinery gases | Smallest molecules. Lowest boiling point. Lowest viscosity. Lightest in colour. |
| Gasoline | |
| Kerosene | |
| Diesel | |
| Fuel oil | |
| Bitumen | Largest molecules. Highest boiling point. Highest viscosity. Darkest in colour. |
A fuel is a substance that, when burned, releases heat energy (exothermic reaction).
Complete Combustion happens when there is enough oxygen available, producing carbon dioxide (CO2) and water (H2O)
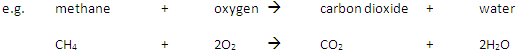
Incomplete Combustion happens when there is not enough oxygen available, with possible products being carbon monoxide (CO), carbon (C, soot), carbon dioxide (CO2) and water (H2O)
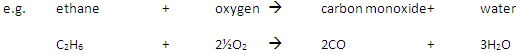
Carbon monoxide may be produced from the incomplete combustion of fuels:
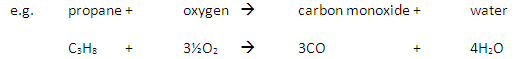
Carbon monoxide is poisonous because it reduces the capacity of the blood to carry oxygen.
When fuels are burned in vehicle engines, high temperatures are reached.
At these high temperatures nitrogen and oxygen from the air react to produce nitrogen oxides:
nitrogen + oxygen → nitrogen oxides
eg
N2 (g) + O2 (g) → 2NO (g)
In the atmosphere these nitrogen oxides can combine with water to produce nitric acid (HNO3).
Fossil fuels such as coal, gas and oil are derived from crude oil.
These fuels are hydrocarbons, but also include impurities such as sulfur.
When the fuels are burned, sulfur dioxide is produced which can escape into the atmosphere:
S (s) + O₂ (g) → SO₂ (g)
Acids formed in the atmosphere can fall as acid rain. This can be a major problem, killing trees and fish in lakes. The acid rain also corrodes limestone buildings and marble statues since these are both made of calcium carbonate (CaCO₃). Some metals such as iron are also attacked by acid rain.
Sulfur dioxide released into the atmosphere from the burning of fossil fuels can react with water and oxygen to make sulfuric acid (H₂SO₄):
2SO₂ (g) + 2H₂O (l) + O₂ (g) → 2H₂SO₄ (aq)
Also, if sulfur dioxide in the atmosphere reacts with just water, a weaker acid called sulfurous acid (H₂SO₃) is formed:
SO₂ (g) + H₂O (l) → H₂SO₃ (aq)
In car engines the temperature is high enough for the nitrogen in the air to react with oxygen to produce oxides of nitrogen, e.g:
N₂ (g) + O₂ (g) → NO₂ (g)
In the atmosphere these nitrogen oxides can produce nitric acid (HNO₃).
Cracking involves the thermal decomposition of long-chain alkanes into shorter-chain alkanes and alkenes:
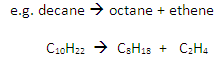
Conditions
Temperature: 600oC
Catalyst: aluminium oxide, Al2O3
Cracking converts long chain hydrocarbons into short chain hydrocarbons.
Long-chain alkanes are broken down into alkanes and alkenes of shorter length.
Crude oil contains a surplus long chains.
Shorter chain hydrocarbons are in greater demand, e.g. petrol.
Cracking also produces alkenes which are used in making polymers and ethanol.