The entire quiz question bank!
This quiz pulls questions at random from the entire 1100+ question-bank
This quiz pulls questions at random from the entire 1100+ question-bank
This quiz pulls questions at random from the entire Double-only question-bank
Please go to The entire quiz question bank (Double only)! to view this quizMake sure you are familiar with units for
Mass: kilogram (kg)
Distance: metre (m)
Speed: metre per second (m/s)
Acceleration: metre per second squared (m/s^2)
Force: newton (N)
Time: second (s)
Gravity: newton/kilogram (N/kg)
the units of:
moment: Newton metre (Nm)
momentum: kilogram metre/second (kgm/s)
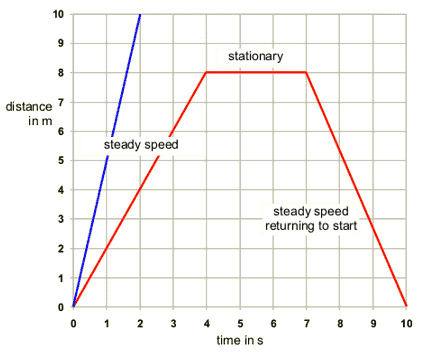
A distance time graph has distance on the y axis (usually in metres) and time on the x axis (usually in seconds). The gradient of the line (change in y/ change in x) is the speed. If the line is flat then the object is stationary.
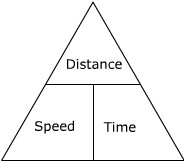
To calculate average speed use
speed (m/s) = distance travelled (m)/ time taken (s)
Apparatus: stop watch and metre rule
mark the start and end positions for the know distance
use a metre rule to measure the distance
line up front of car with start point, release and start timer
move eyes to end point
stop timer when front of car passes end point
improve by repeating and averaging
make sure car starts from stationary
calculate average speed using : average speed = distance travelled/ time taken
on a velocity time graph the velocity-time graph the velocity is on the y axis (usually in m/s) and time is on the x axis (usually in s). If the line is flat then the object is moving at a constant velocity. the gradient of the line is the acceleration. The area under the line is the distance travelled.
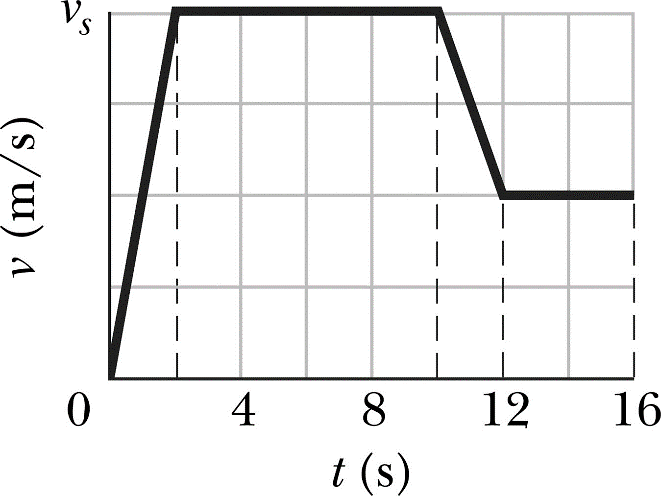
Gradient = acceleration= change in y/ change in x = change in velocity/time
The area under the graph can be calculated as rectangles and triangles, or by counting boxes, is equal to the distance travelled.
v2=u2+2as
v= final speed
u= initial speed
a= acceleration
s= distance moved
see all the rearrangements of this equation.
Forces can act on a body to change the velocity, so the speed, direction or both.
Or forces can change the shape of a body, stretching it squishing it or twisting it.
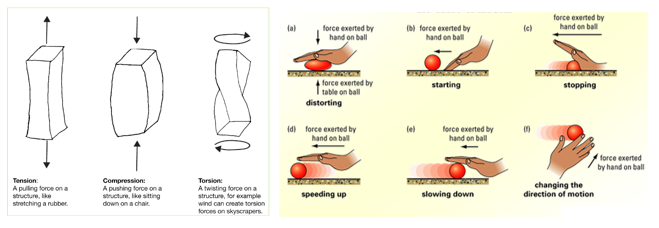
different types of forces include:
Gravitational, weight, friction, electrostatic, air resistance (drag), tension (force in a spring), up thrust, lift, thrust
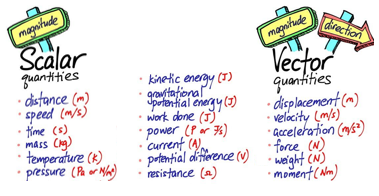
scalars are quantities with only magnitude (size)
vectors are quantities with magnitude (size) and direction
Force has a magnitude measured in (N) but it also has a direction, a push or a pull, up, down, left or right. So force is a vector.
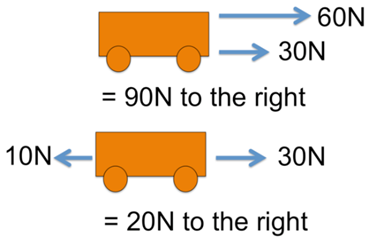
Forces along a line can combine by addition.
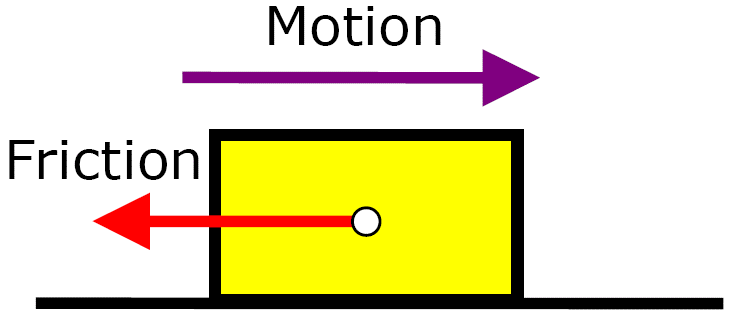
Friction is caused by surfaces rubbing. The force always acts in the opposite direction to motion.
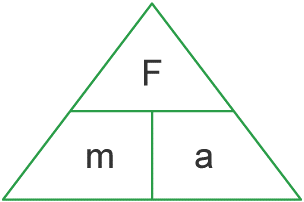
Force = Mass x Acceleration.
the force refers to the resultant force or the combined forces as seen in 1.15
Weight (N)= Mass (kg) x gravitational field strength (N/kg)
gravitational field strength on earth is approx. 10 N/kg and in GCSEs is taken to be 10 N/kg.
Stopping distance = Thinking distance + Breaking distance
Thinking distance Affected by:
Tiredness
Alcohol
speed of the car
Drugs (avoid as drugs can increase or decrease thinking distance)
Braking distance affected by:
Road conditions
Tyre conditions
Brake conditions
speed of the car
mass of the car
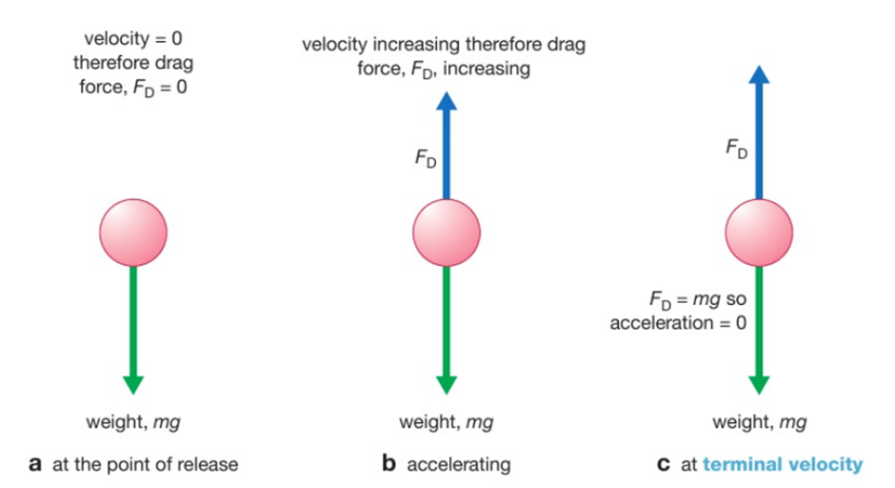
Initially the only force is weight as drag is proportional to velocity. So the object accelerates downwards. As it accelerates the velocity so the drag increases as well. meaning there is a smaller resultant force downwards so a smaller acceleration. Until the object reaches a speed where the drag is equal to the weight meaning there is no acceleration, this velocity is know as terminal velocity.
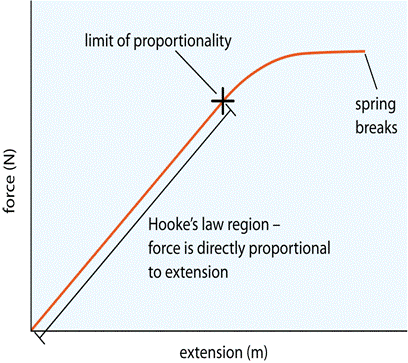
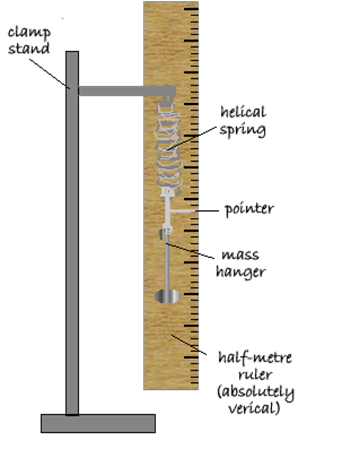
Hooke’s law is that extension is directly proportional to force applied. This is shown by the straight line on the force-extension graph. Hooke’s law is obeyed as long as the line is straight.

Elastic behaviour is the ability of a material to recover original shape after the force is removed. in a spring this occurs when the force is lower than the elastic limit. loading and unloading force extension curves can be different as long as it returns to its original shape.
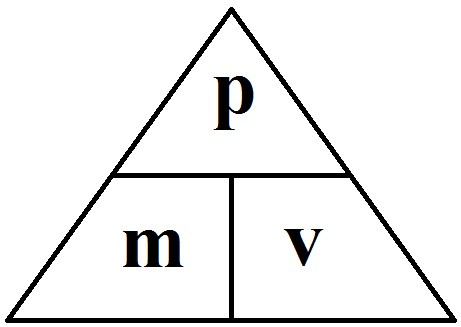
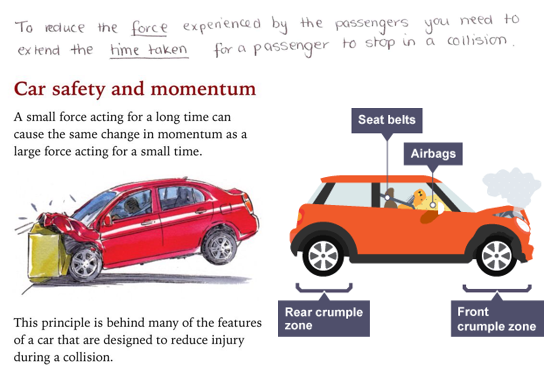
momentum (kgm/s)= mass (kg) x velocity (m/s)
To reduce the force experienced by the passenger you need to extend the time for a passenger to stop in a collision. As force is the change in momentum divided by time.

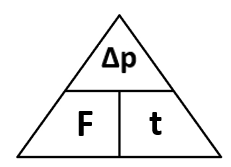
Force is the rate of change of momentum. So Force (N) = change in momentum (kgm/s) / time (s)
Every action has an equal and opposite reaction.
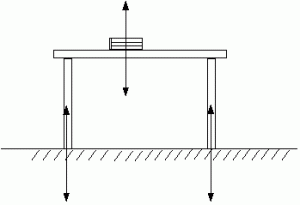
Book pushes down on table, table pushes up on book. So book doesn’t accelerate.
Table pushes down on floor, floor pushes up on table. So table doesn’t accelerate.