Alkanes & Crude Oil quiz
A good way to remember the names of organic molecules is to make up a silly mnemonic where the first letter of each word matches the first letter of the organic molecules. For example the first 10 alkanes in order are , Methane, Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane and Decane. These can be memorised with “Many elephants prefer blue pinapples. However hungry orangutans never do.” This isn’t a very good mnemonic, but it is the one I made up for myself when I was at school, and you are best making up your own. The sillier, smellier and more colourful the better.
Example:
Sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
Word equation:
sodium + water –> sodium hydroxide + hydrogen
Writing the chemical equation
A chemical equation represents what happens in terms of atoms in a chemical reaction.
Step 1: To write a chemical equation we need to know the chemical formulae of the substances.
Na + H2O –> NaOH + H2
Step 2: The next step is to balance the equation: write a large number before each compound so the number of atoms of each element on the left hand side (reactants) matches the number on the right (products). This large number is the amount of each compound or element.
During this balancing stage the actual formulas for each compound must not be changed. Only the number of each compound changes.
2Na + 2H2O –> 2NaOH + H2
If asked for an equation, the chemical equation must be given.
State symbols are used to show what physical state the reactants and products are in.
| State symbols | Physical state |
|---|---|
| (s) | Solid |
| (l) | Liquid |
| (g) | Gas |
| (aq) | Aqueous solution (dissolved in water) |
Example:
A solid piece of sodium (Na) reacts with water (H2O) to produce a solution of sodium hydroxide (NaOH) and hydrogen gas (H2).
2Na(s) + 2H2O(l) –> 2NaOH(aq) + H2(g)
Carbon dioxide (CO2) has a simple molecular structure. This just means that it is made up of molecules.
Within each molecule are atoms bonded to each other covalently. These covalent bonds inside the molecules are strong.
However, between the molecules are weak forces of attraction that require little energy to break. These forces are not covalent bonds. This is why simple molecular substances such as carbon dioxide have a low boiling point.
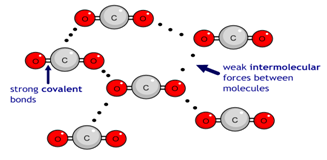
So when carbon dioxide changes from a solid to a gas, for example, that process can be represented as:
CO₂ (s) → CO₂ (g)
Notice that even though there has been a dramatic change of state from solid to gas, the substance before and after the change is always made up of carbon dioxide molecules. During the change of the state the covalent bonds within each molecule remain unbroken. Instead it is the weak forces of attraction between the molecules which have been overcome.
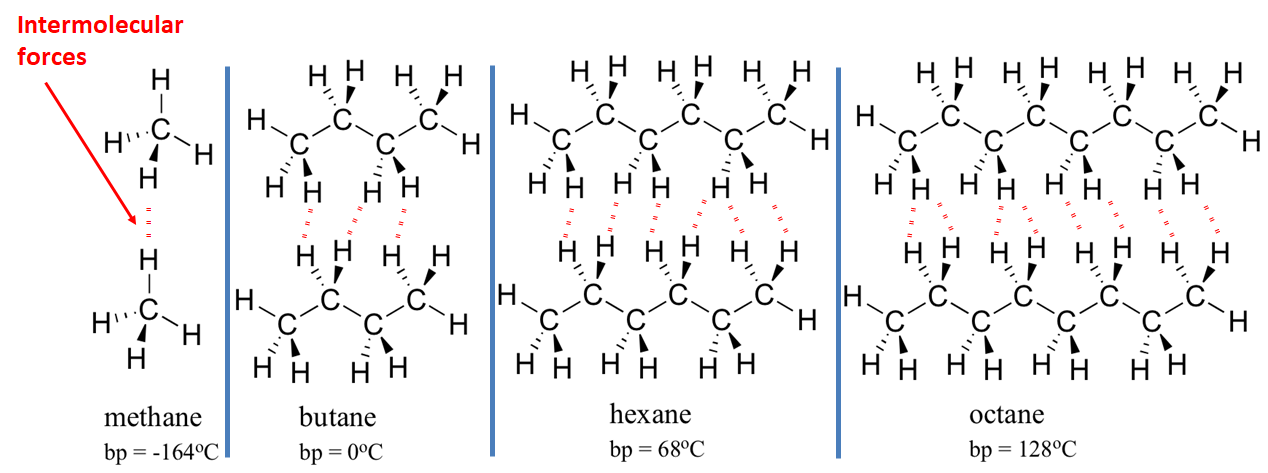
Larger molecules tend to have higher boiling points.
This is because larger molecules (molecules with more mass) have more forces of attraction between them. These forces, although weak, must be overcome if the substance is to boil, and larger molecules have more attractions which must be overcome.
Carbon dioxide (CO2) is a greenhouse gas.
It absorbs infra-red radiation and therefore warms the atmosphere. This leads to global warming.
This may cause climate change.
Hydrocarbon: A molecule containing only hydrogen and carbon
The molecular formula shows the actual number of atoms of each element in a molecule.
The general formula shows the relationship between the number of atoms of one element to another within a molecule. Members of a homologous series share the same general formula. The general formula for alkanes is CnH2n+2 and the general formula for alkenes is CnH2n.
A structural formula shows how the atoms in a molecule are joined together.
The displayed formula is a full structural formula which shows all the bonds in a molecule as individual lines.
The terms above are demonstrated with the example of butane.
The terms above are demonstrated with the example of ethene, which contains a double bond.
The molecular formula shows the actual number of atoms of each element in a molecule.
The empirical formula shows the simplest whole number ratio of atoms present in a compound. So the molecular formula is a multiple of the empirical formula.
The general formula shows the relationship between the number of atoms of one element to another within a molecule. Members of a homologous series share the same general formula. The general formula for alkanes is CnH2n+2 and the general formula for alkenes is CnH2n.
A structural formula shows how the atoms in a molecule are joined together.
The displayed formula is a full structural formula which shows all the bonds in a molecule as individual lines.
The terms above are demonstrated with the example of butane.
The terms above are demonstrated with the example of ethene, which contains a double bond.
Isomers are molecules with the same molecular formula but with a different structure.
The names of organic molecules are based on the number of carbon atoms in the longest chain. This chain is the longest consecutive line of carbon atoms, even if this line bends.
| The name is based on the number of carbon atoms in the longest chain |
|---|
| 1 Meth- |
| 2 Eth- |
| 3 Prop- |
| 4 But- |
| 5 Pent- |
| 6 Hex- |
| 7 Hept- |
| 8 Oct- |
| 9 Non- |
| 10 Dec- |
Hydrocarbons are molecules which contain only hydrogen and carbon.
Naming straight-chain alkanes
The simplest hydrocarbons are alkanes. They contain only single bonds, and have “-ane” in the name.
For example, the displayed formula of ethane (C₂H₆) is:
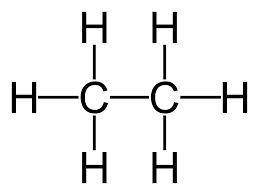
The name “ethane” contains “eth-” because there are 2 carbon atoms in the longest chain, and the name contains “-ane” because the molecule only has single bonds so is an alkane.
Another example is pentane (C₅H₁₂) which has the displayed formula:
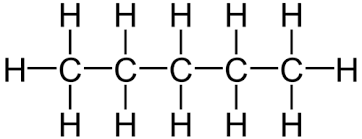
The name “pentane” contains “pent-” because there are 5 carbon atoms in the longest chain, and the name contains “-ane” because the molecule only has single bonds, so is an alkane.
Remember, it does not matter if the longest consecutive line of carbons bends around. For example the displayed formula below still shows a very normal molecule of pentane (5 carbons in a row). Pentane is not normally drawn with the longest chain of carbons bent around because it could be confusing.
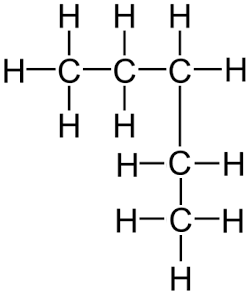
You might also see the bonds drawn at angles. Don’t worry, the displayed formula below is still pentane, as can be seen by the fact there are 5 carbon atoms in the longest chain, surrounded by hydrogen atoms bonded to the carbon atoms by single bonds.
A shorter way to express the detailed structure of an organic molecule is the structural formula. The structural formula for pentane is CH₃-CH₂-CH₂-CH₂-CH₃, which tells us the same information about the molecule as does the displayed formula, without the hassle of having to draw all the bonds or all the hydrogen atoms.
Naming straight-chain alkenes
Another simple group of hydrocarbons is the alkenes. They contain a carbon-to-carbon double bond, which also means they have two fewer hydrogen atoms than their corresponding alkane. An alkene has “-ene” in its name.
For example, the displayed formula for ethene (C₂H₄) is:
and the displayed formula of propene (C₃H₆) is:
With longer alkene molecules the double bond might appear in different locations of the carbon chain, so the name needs to be a little bit more complicated to be able to describe these differences clearly. A number is added in the middle of the name to indicate at which carbon the double bond starts.
So the displayed formula of pent-1-ene is:
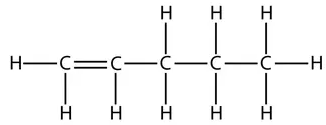
and this is the displayed formula of pent-2-ene:
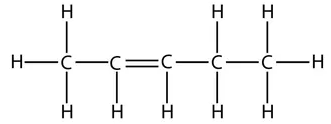
However, take care that when counting which carbon has the double bond. The numbers start from the end that produces the smallest numbers in the name. For example, this is the displayed formula for pent-1-ene again, but just drawn the other way round. It is still pent-1-ene (you can’t get pent-4-ene):
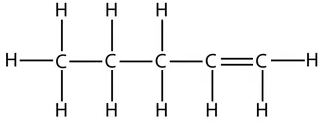
Naming straight-chain alcohols
We get the same pattern all over again with the group of organic molecules called alcohols, which are recognised by an -OH functional group. For example here is the displayed formula for ethanol, which has 2 carbon atoms in the longest chain:
and here is the displayed formula for butanol:
Summary of naming simple straight-chain organic molecules
The following table summarises the naming of some of the straight-chain alkanes, alkenes and alcohols, giving a name and a molecular formula for each:
| Carbons in longest chain | Alkanes | Alkenes | Alcohols |
|---|---|---|---|
| 1 | methane, CH₄ | - | methanol, CH₄O |
| 2 | ethane, C₂H₆ | ethene, C₂H₄ | ethanol, C₂H₆O |
| 3 | propane, C₃H₈ | propene, C₃H₆ | propanol, C₃H₈O |
| 4 | butane, C₄H₁₀ | butene, C₄H₈ | butanol, C₄H₁₀O |
Naming branched alkanes and alkenes
The naming conventions for organic molecules cover more than the straight chain molecules. Branched molecules are named depending on the number of carbon atoms in the branch. A branch with 1 carbon is called “methyl” and a branch with 2 carbons is called “ethyl”. This is similar to the conventions covered above, plus the “-yl-” bit just says it is a branch.
For example, this is the displayed formula for 2-methyl hexane:
In the name 2-methyl hexane, the number 2 indicates that when counting along the longest carbon chain the methyl branch comes off the second carbon atom. The “methyl” bit of the name says there is one branch of 1 carbon. The “hex” bit of the name says the longest consecutive chain of carbon atoms is 6. The “ane” bit says the molecule has only single bonds.
When counting along the carbon atoms of the longest chain to work out the name, the numbering of carbon atoms starts from the end nearest to the branch. Another way to put this is that the name is given such that the numbers in the name are as low as possible. For example, here is the displayed formula for 4-ethyl octane:
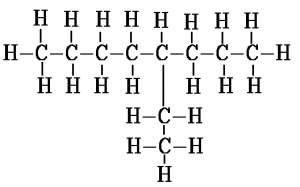
Another example, this time with 2 methyl branches coming off the second and third carbons of the chain, is 2,3-dimethyl hexane. The “di” in the name indicates there are two methyl groups. This is the same way in which “di” indicates there are two oxygen atoms in carbon dioxide.
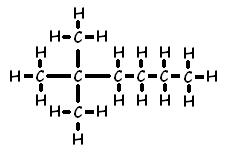
Another example of how the naming convention works for branches is 2,2-dimethyl hexane:
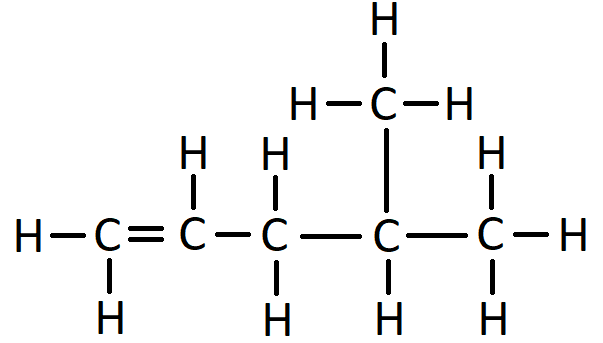
This naming of branches also applies to alkenes. Here is the displayed formula of 4-methylpent-1-ene:
The molecular formula describes the actual number of each type of each atom in a molecule.
For example, a molecular formula of C₆H₁₂ tells us that in each molecule there are 6 carbon atoms and 12 hydrogen atoms.
However, the molecular formula tells us nothing about how those atoms are arranged. For example, it does not tell us if there any branches of carbon atoms coming off the main carbon-carbon chain, nor how long or how many there might be.
On the other hand, the structural formula and displayed formula of a molecule tell us clearly how the atoms are arranged in that molecule.
This means that if we are given a molecular formula only, there may be several possible structural and displayed formulae all of which could apply for that molecule.
When trying to work out possible structural or displayed formulae from a molecular formula there are several clues:
Crude Oil is a mixture of hydrocarbons.
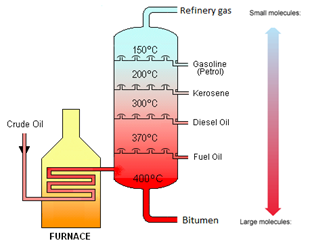
This video does not quite use the right language for the various fractions as appropriate to the Edexcel iGCSE, but it is nevertheless a good description of the process. Make sure you use the notes on tutorMyself.com to get the exact language you will need for your exam.
And another somewhat older video showing the industrial process of fractional distillation:
Crude oil is separated into fractions by the process of fractional distillation.
| Fraction | Use |
|---|---|
| Refinery gases | Bottled gas |
| Gasoline | Fuel for cars |
| Kerosene | Fuel for aeroplanes |
| Diesel Oil | Fuel for lorries |
| Fuel Oil | Fuel for ships |
| Bitumen | Road Surfacing |
The boiling point increases as the number of carbon atoms (chain length) increases.
The viscosity increases as the number of carbon atoms (chain length) increases.
The greater the number of carbon atoms (chain length), the darker in colour that fraction is.
The viscosity of a fluid describes how easily it flows. Water has a low viscosity, it flows very easily. Crude oil has a higher viscosity than water, it does not flow very easily.
| Fractions (in order) | Properties |
|---|---|
| Refinery gases | Smallest molecules. Lowest boiling point. Lowest viscosity. Lightest in colour. |
| Gasoline | |
| Kerosene | |
| Diesel | |
| Fuel oil | |
| Bitumen | Largest molecules. Highest boiling point. Highest viscosity. Darkest in colour. |
A fuel is a substance that, when burned, releases heat energy (exothermic reaction).
Complete Combustion happens when there is enough oxygen available, producing carbon dioxide (CO2) and water (H2O)
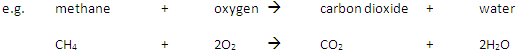
Incomplete Combustion happens when there is not enough oxygen available, with possible products being carbon monoxide (CO), carbon (C, soot), carbon dioxide (CO2) and water (H2O)
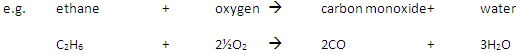
Carbon monoxide may be produced from the incomplete combustion of fuels:
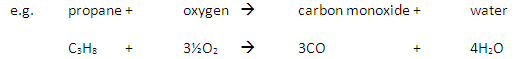
Carbon monoxide is poisonous because it reduces the capacity of the blood to carry oxygen.
When fuels are burned in vehicle engines, high temperatures are reached.
At these high temperatures nitrogen and oxygen from the air react to produce nitrogen oxides:
nitrogen + oxygen → nitrogen oxides
eg
N2 (g) + O2 (g) → 2NO (g)
In the atmosphere these nitrogen oxides can combine with water to produce nitric acid (HNO3).
Fossil fuels such as coal, gas and oil are derived from crude oil.
These fuels are hydrocarbons, but also include impurities such as sulfur.
When the fuels are burned, sulfur dioxide is produced which can escape into the atmosphere:
S (s) + O₂ (g) → SO₂ (g)
Acids formed in the atmosphere can fall as acid rain. This can be a major problem, killing trees and fish in lakes. The acid rain also corrodes limestone buildings and marble statues since these are both made of calcium carbonate (CaCO₃). Some metals such as iron are also attacked by acid rain.
Sulfur dioxide released into the atmosphere from the burning of fossil fuels can react with water and oxygen to make sulfuric acid (H₂SO₄):
2SO₂ (g) + 2H₂O (l) + O₂ (g) → 2H₂SO₄ (aq)
Also, if sulfur dioxide in the atmosphere reacts with just water, a weaker acid called sulfurous acid (H₂SO₃) is formed:
SO₂ (g) + H₂O (l) → H₂SO₃ (aq)
In car engines the temperature is high enough for the nitrogen in the air to react with oxygen to produce oxides of nitrogen, e.g:
N₂ (g) + O₂ (g) → NO₂ (g)
In the atmosphere these nitrogen oxides can produce nitric acid (HNO₃).
Alkanes have the general formula CnH2n+2
This means that to work out the number of hydrogens, you double the number of carbons and then add two.
Saturated: A molecule containing only single bonds between carbon atoms. For example, alkanes as described as saturated molecules.
Unsaturated: A molecule containing a carbon-carbon double or triple bond. For example, alkenes as described as unsaturated molecules.
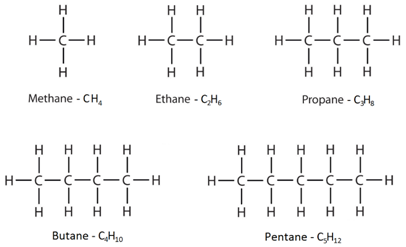
The displayed formulae show all the atoms and bonds drawn out.
The molecular formulae just show the number of each type of atom in the molecule.
This video is a good introduction to alkanes and crude oil: