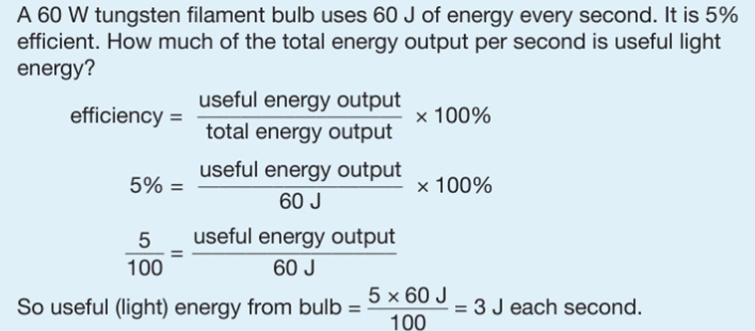
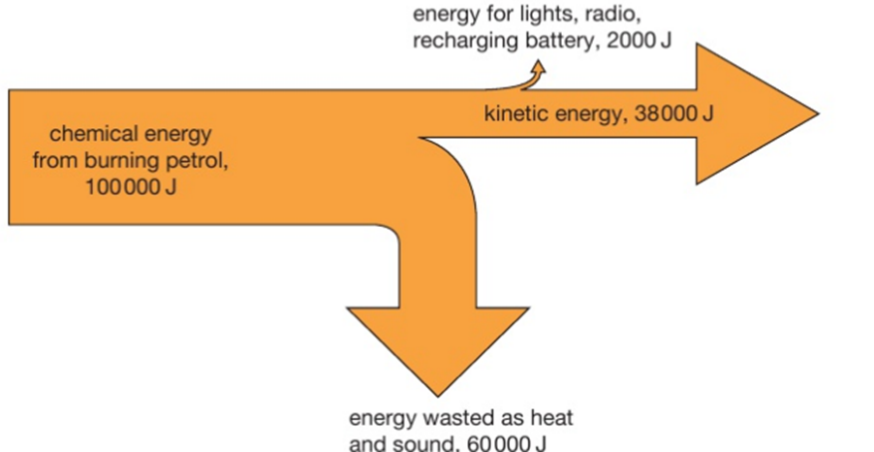
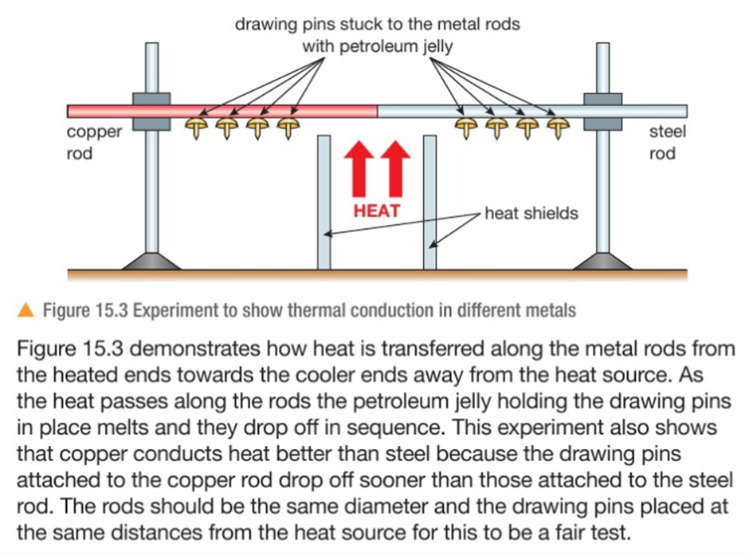
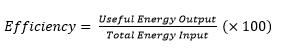4.02 describe energy transfers involving energy stores: energy stores: chemical, kinetic, gravitational, elastic, thermal, magnetic, electrostatic, nuclear and energy transfers: mechanically, electrically, by heating, by radiation (light and sound)
|
Energy Stores: Chemical – e.g. the food we eat Kinetic – movement energy Gravitational – objects that are lifted up Elastic – e.g. from springs Thermal – from hot objects Magnetic – objects in magnetic fields Electrostatic – charged objects Nuclear – stored within a nucleus
|