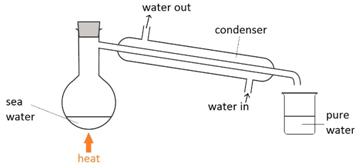
Simple distillation
This method is used to separate a liquid from a solution. For example: separating water from salt water.
The salt water is boiled. The water vapour condenses back into a liquid when passed through the condenser. The salt is left behind in the flask.
Note: cold water is passed into the bottom of the condenser and out through the top so that the condenser completely fills up with water.
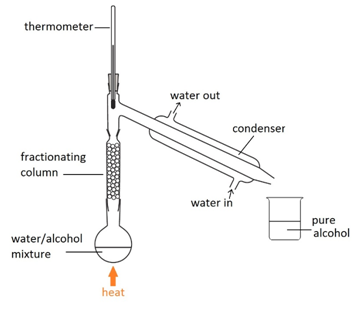
Fractional distillation
This method is used to separate a mixture of different liquids that have different boiling points. For example, separating alcohol from a mixture of alcohol and water.
Water boils at 100oC and alcohol boils at 78oC. By using the thermometer to carefully control of temperature of the column, keeping it at 78oC, only the alcohol remains as vapour all the way up to the top of the column and passes into the condenser.
The alcohol vapours then condense back into a liquid.
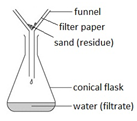 Filtration
Filtration
This method is used to separate an insoluble solid from a liquid. For example: separating sand from a mixture of sand and water.
The mixture is poured into the filter paper. The sand does not pass through and is left behind (residue) but the water passes through the filter paper and is collected in the conical flask (filtrate).
Crystallisation
This method is used to obtain a salt which contains water of crystallisation from a salt solution. For example: hydrated copper sulfate crystals (CuSO4.5H2O(s)) from copper sulfate solution (CuSO4(aq)).
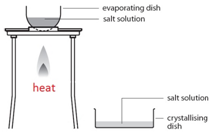 Gently heat the solution in an evaporating basin to evaporate some of the water
Gently heat the solution in an evaporating basin to evaporate some of the water- until crystals form on a glass rod (which shows that a hot saturated solution has formed).
- Leave to cool and crystallise.
- Filter to remove the crystals.
- Dry by leaving in a warm place.
If instead the solution is heated until all the water evaporates, you would produce a powder of anhydrous copper sulfate (CuSO4(s)).
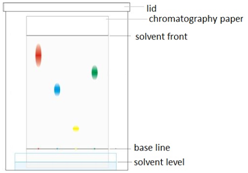
Paper chromatography
This method can be used to separate the parts of a mixture into their components. For example, the different dyes in ink can all be separated and identified.
The coloured mixture to be separated (e.g. a food dye) is dissolved in a solvent like water or ethanol and carefully spotted onto the chromatography paper on the baseline, which is drawn in pencil so it doesn’t ‘run or smudge’.
The paper is carefully dipped into the solvent and suspended so the baseline is above the liquid solvent, otherwise all the spots would dissolve in the solvent. The solvent is absorbed into the paper and rises up it as it soaks into the paper. The choice of solvent depends on the solubility of the dye. If the dye does not dissolve in water then normally an organic solvent (e.g. ethanol) is used.
As the solvent rises up the paper it will carry the dyes with it. Each different dye will move up the paper at different rates depending on how strongly they stick to the paper and how soluble they are in the solvent.