1:08 understand how to classify a substance as an element, a compound or a mixture
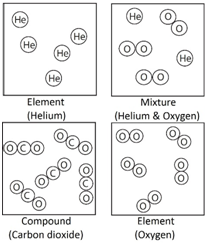
Element: The simplest type of substances made up of only one type of atom.
Compound: A substance that contains two or more elements chemically joined together in fixed proportions.
Mixture: Different substances in the same space, but not chemically combined.
Note: elements such as oxygen (O2) are described as diatomic because they contain two atoms.
The full list of elements that are diatomic is:
- Hydrogen (H2)
- Nitrogen (N2)
- Fluorine (F2)
- Oxygen (O2)
- Iodine (I2)
- Chlorine (Cl2)
- Bromine (Br2)