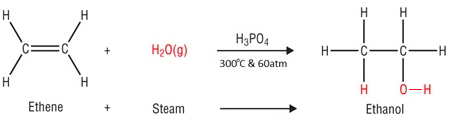
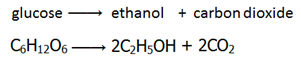 In the hydration of ethene, ethanol is made by passing ethene and steam over a catalyst.
In the hydration of ethene, ethanol is made by passing ethene and steam over a catalyst.
Water is added to ethene, this is known as hydration.
Conditions
Catalyst: Phosphoric acid (H3PO4)
Temperature: 300°C
Pressure: 60 atm
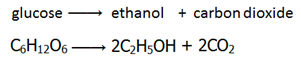 Fermentation is the conversion of sugar, e.g. glucose into ethanol by enzymes from yeast.
Fermentation is the conversion of sugar, e.g. glucose into ethanol by enzymes from yeast.
Conditions
Catalyst: Zymase (enzyme found in yeast)
Temperature: 30°C – The process is carried out at low temperatures as not to denature the enzymes.
Other: Anaerobic (no oxygen present) – if oxygen were present, the yeast produce carbon dioxide and water instead of ethanol.
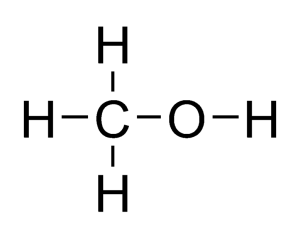
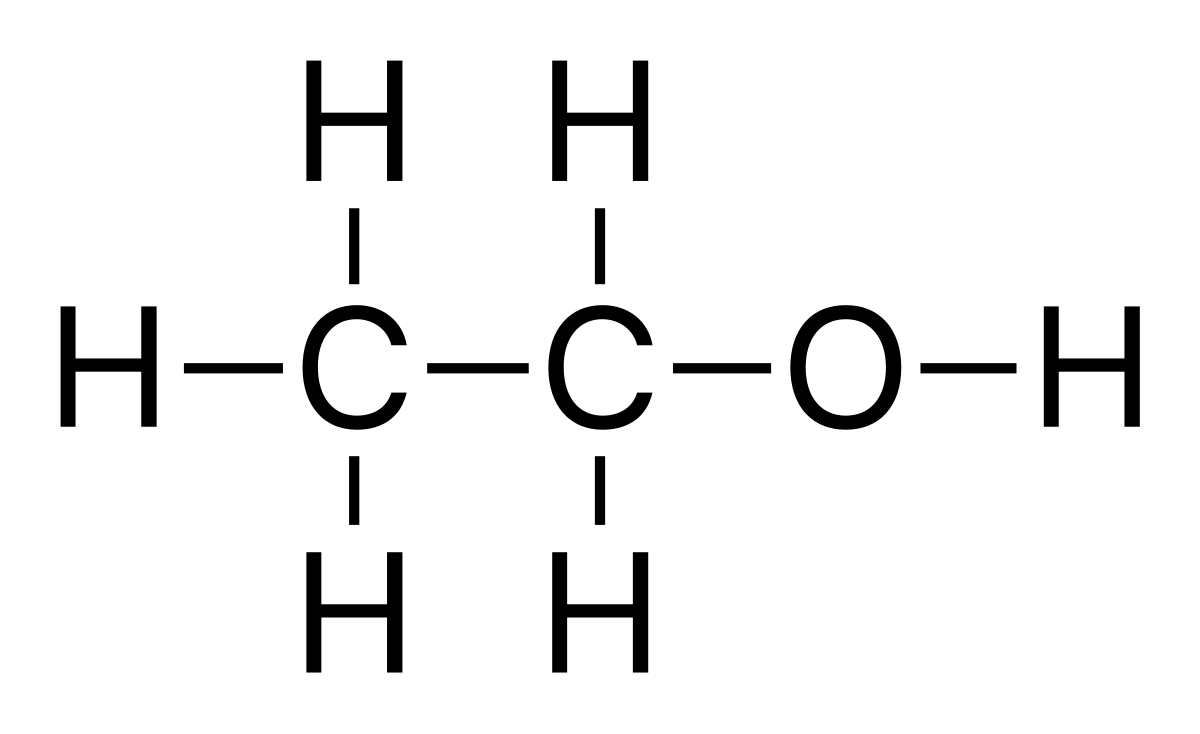
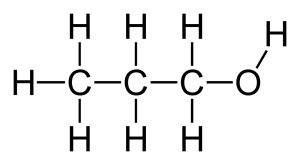
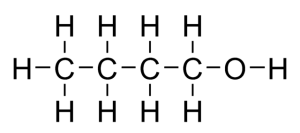
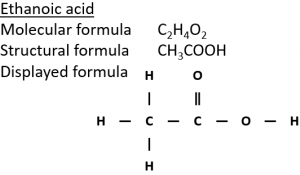
 In the
In the