2:23 (Triple only) explain how the method of extraction of a metal is related to its position in the reactivity series, illustrated by carbon extraction for iron and electrolysis for aluminium
Extraction of a metal from its ore typically involves removing oxygen from metal oxides.
If the ore contains a metal which is below carbon in the reactivity series then the metal is extracted by reaction with carbon in a displacement reaction.
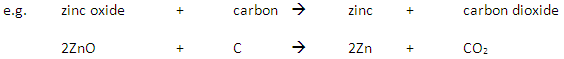
If the ore contains a metal which is above carbon in the reactivity series then electrolysis (or reaction with a more reactive metal) is used to extract the metal.