3:06 (Triple only) know that bond-breaking is an endothermic process and that bond-making is an exothermic process
During chemical reactions, the bonds in the reactants must be broken, and new ones formed to make the products.
Breaking bonds need energy and therefore is described as endothermic.
Energy is released when new bonds are made and therefore is described as exothermic.
If bonds are both broken and made during chemical reactions, why can a reaction overall be describe as either exothermic or endothermic?
Example: hydrogen reacts with oxygen producing water. Overall energy is released and therefore the reaction is exothermic.
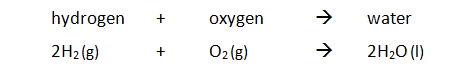
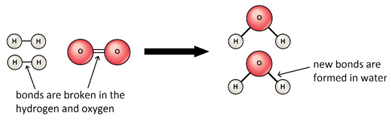
The reaction is exothermic because the energy needed to break the bonds is less than the energy released in making new bonds.
If a reaction is endothermic then the energy needed to break the bonds is more than the energy released in making new bonds.