mass = Mr x moles

https://www.allnetwork.org/kontak/
A good way to remember the names of organic molecules is to make up a silly mnemonic where the first letter of each word matches the first letter of the organic molecules. For example the first 10 alkanes in order are , Methane, Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane and Decane. These can be memorised with “Many elephants prefer blue pinapples. However hungry orangutans never do.” This isn’t a very good mnemonic, but it is the one I made up for myself when I was at school, and you are best making up your own. The sillier, smellier and more colourful the better.
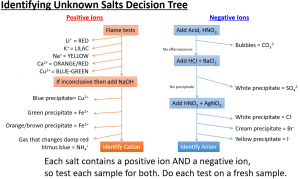
Here is summary of “testing for ions” (otherwise known as qualitative analysis). These “tests for ions” are commonly asked in iGCSE Edexcel Chemistry.
Each ionic compound contains positive ions and negative ions. To use the decision tree, start at the top and if a test is negative then move down to the next test.
| Spec | Q'stion | Ans | MS |
|---|