1:12 understand how to use the calculation of Rf values to identify the components of a mixture
When analysing a chromatogram, the mixture being analysed is compared to standard reference materials by measuring how far the various dyes have travelled up the paper from the baseline where they started.
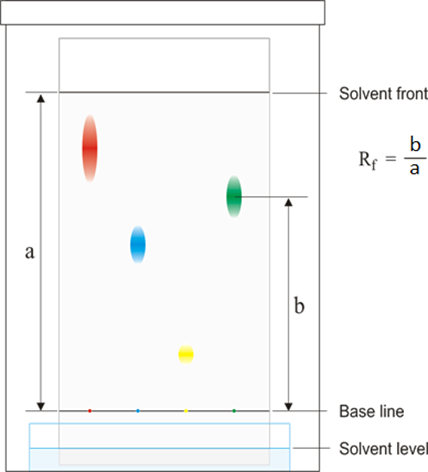
For each dye, the Rf value is calculated. To do this, 2 distances are measured:
- The distance between the baseline and the dye
- The distance between the baseline and the solvent front, which is how far the solvent has travelled from the baseline
The Rf value is calculated as follows:
If the Rf value of one of the components of the mixture equals the Rf value of one of the standard reference materials then that component is know to be that reference material.
Note that because the solvent always travels at least as far as the highest dye, the Rf value is always between 0 and 1.
Dyes which are more soluble will have higher Rf values than less soluble dyes. In other words, more soluble dyes move further up the paper. The extreme case of this is for insoluble dyes which don't move at all (Rf value = 0). The other aspect affecting how far a dye travels is the affinity that dye has for the paper (how well it 'sticks' to the paper).