3:03 calculate the heat energy change from a measured temperature change using the expression Q = mcΔT
Calorimetry allows for the measurement of the amount of energy transferred in a chemical reaction to be calculated.
EXPERIMENT1: Displacement, dissolving and neutralisation reactions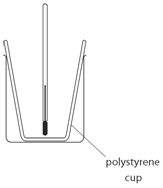
Example: magnesium displacing copper from copper(II) sulfate
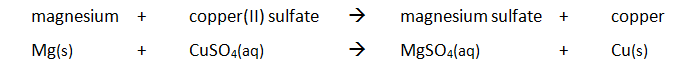
Method:
- 50 cm3 of copper(II) sulfate is measured and transferred into a polystyrene cup.
- The initial temperature of the copper sulfate solution is measured and recorded.
- Magnesium is added and the maximum temperature is measured and recorded.
- The temperature rise is then calculated. For example:
| Initial temp. of solution (oC) | Maximium temp. of solution (oC) | Temperature rise (oC) |
|---|---|---|
| 24.2 | 56.7 | 32.5 |
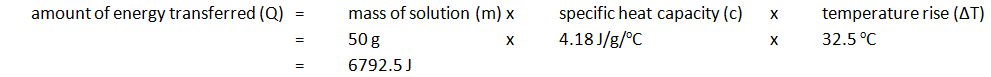
Note: mass of 50 cm3 of solution is 50 g
EXPERIMENT2: Combustion reactions
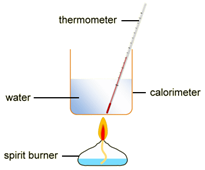
To measure the amount of energy produced when a fuel is burnt, the fuel is burnt and the flame is used to heat up some water in a copper container
Example: ethanol is burnt in a small spirit burner
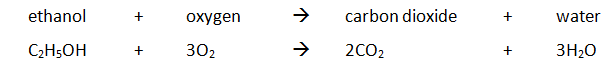
Method:
- The initial mass of the ethanol and spirit burner is measured and recorded.
- 100cm3 of water is transferred into a copper container and the initial temperature is measured and recorded.
- The burner is placed under of copper container and then lit.
- The water is stirred constantly with the thermometer until the temperature rises by, say, 30 oC
- The flame is extinguished and the maximum temperature of the water is measured and recorded.
- The burner and the remaining ethanol is reweighed. For example:
| Mass of water (g) | Initial temp of water (oC) | Maximum temp of water (oC) | Temperature rise (oC) | Initial mass of spirit burner + ethanol (g) | Final mass of spirit burner + ethanol (g) | Mass of ethanol burnt (g) |
|---|---|---|---|---|---|---|
| 100 | 24.2 | 54.2 | 30.0 | 34.46 | 33.68 | 0.78 |
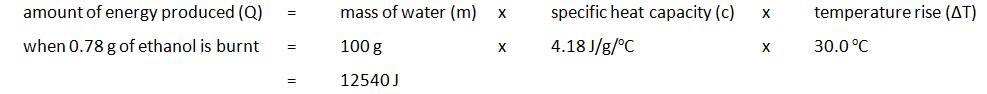
The amount of energy produced per gram of ethanol burnt can also be calculated:
