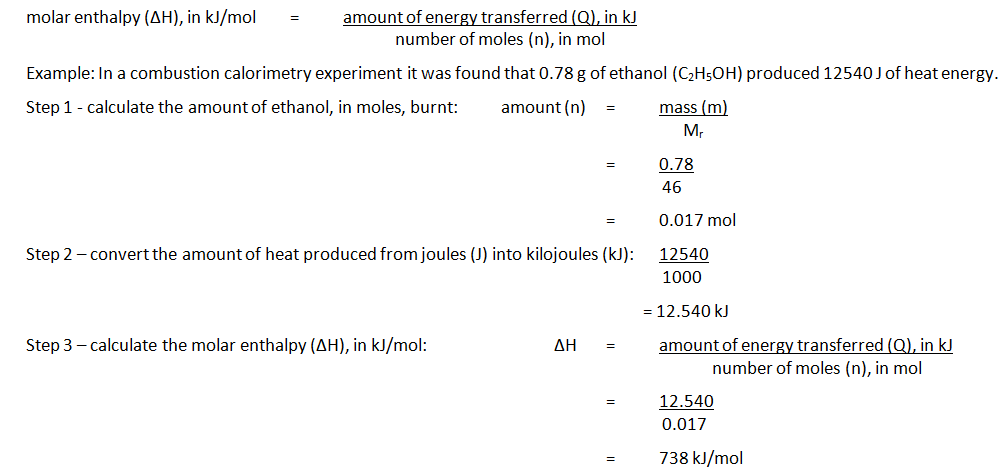
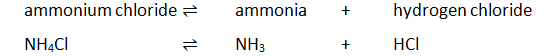The names of organic molecules are based on the number of carbon atoms in the longest chain. This chain is the longest consecutive line of carbon atoms, even if this line bends.
| The name is based on the number of carbon atoms in the longest chain |
| 1 Meth- |
| 2 Eth- |
| 3 Prop- |
| 4 But- |
| 5 Pent- |
| 6 Hex- |
| 7 Hept- |
| 8 Oct- |
| 9 Non- |
| 10 Dec- |
Hydrocarbons are molecules which contain only hydrogen and carbon.
Naming straight-chain alkanes
The simplest hydrocarbons are alkanes. They contain only single bonds, and have “-ane” in the name.
For example, the displayed formula of ethane (C₂H₆) is:
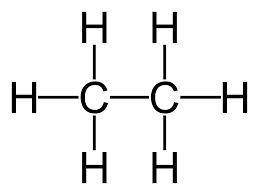
The name “ethane” contains “eth-” because there are 2 carbon atoms in the longest chain, and the name contains “-ane” because the molecule only has single bonds so is an alkane.
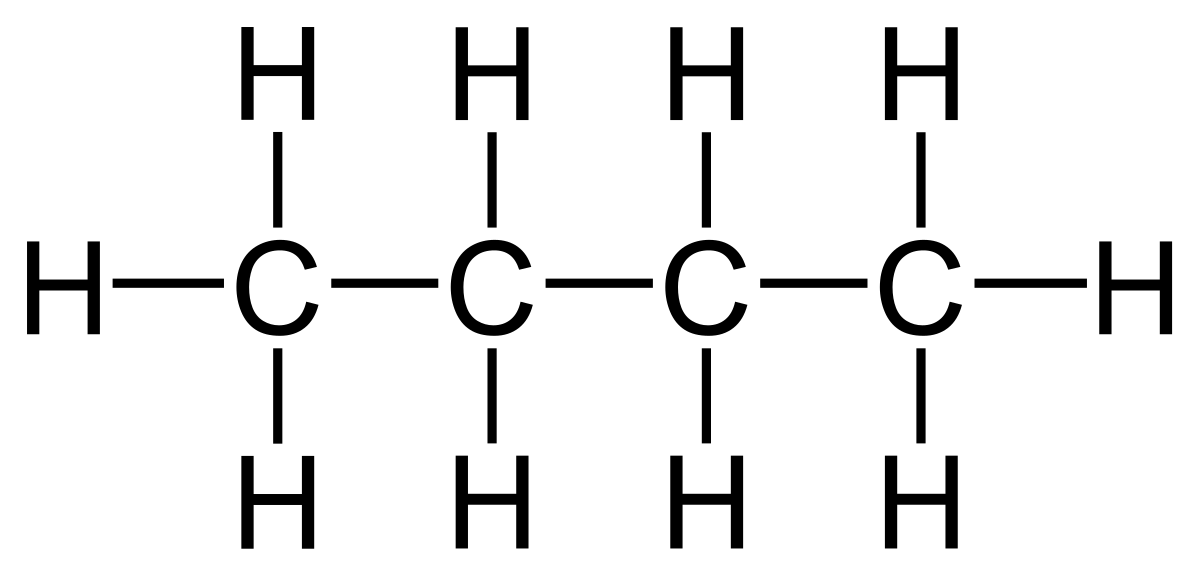
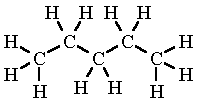
Another example is pentane (C₅H₁₂) which has the displayed formula:
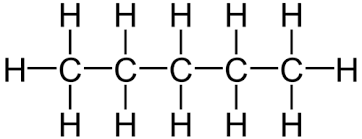
The name “pentane” contains “pent-” because there are 5 carbon atoms in the longest chain, and the name contains “-ane” because the molecule only has single bonds, so is an alkane.
Remember, it does not matter if the longest consecutive line of carbons bends around. For example the displayed formula below still shows a very normal molecule of pentane (5 carbons in a row). Pentane is not normally drawn with the longest chain of carbons bent around because it could be confusing.
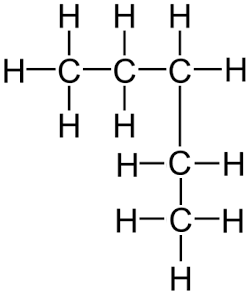
You might also see the bonds drawn at angles. Don’t worry, the displayed formula below is still pentane, as can be seen by the fact there are 5 carbon atoms in the longest chain, surrounded by hydrogen atoms bonded to the carbon atoms by single bonds.

A shorter way to express the detailed structure of an organic molecule is the structural formula. The structural formula for pentane is CH₃-CH₂-CH₂-CH₂-CH₃, which tells us the same information about the molecule as does the displayed formula, without the hassle of having to draw all the bonds or all the hydrogen atoms.
Naming straight-chain alkenes
Another simple group of hydrocarbons is the alkenes. They contain a carbon-to-carbon double bond, which also means they have two fewer hydrogen atoms than their corresponding alkane. An alkene has “-ene” in its name.
For example, the displayed formula for ethene (C₂H₄) is:

and the displayed formula of propene (C₃H₆) is:

With longer alkene molecules the double bond might appear in different locations of the carbon chain, so the name needs to be a little bit more complicated to be able to describe these differences clearly. A number is added in the middle of the name to indicate at which carbon the double bond starts.
So the displayed formula of pent-1-ene is:
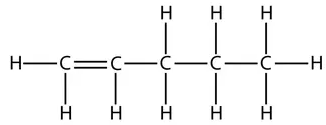
and this is the displayed formula of pent-2-ene:
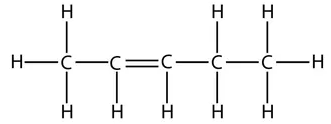
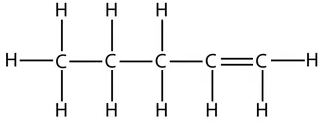
However, take care that when counting which carbon has the double bond. The numbers start from the end that produces the smallest numbers in the name. For example, this is the displayed formula for pent-1-ene again, but just drawn the other way round. It is still pent-1-ene (you can’t get pent-4-ene):
Naming straight-chain alcohols
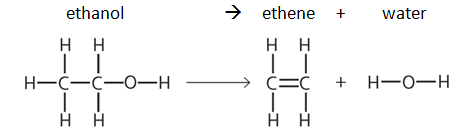
We get the same pattern all over again with the group of organic molecules called alcohols, which are recognised by an -OH functional group. For example here is the displayed formula for ethanol, which has 2 carbon atoms in the longest chain:

and here is the displayed formula for butanol:

Summary of naming simple straight-chain organic molecules
The following table summarises the naming of some of the straight-chain alkanes, alkenes and alcohols, giving a name and a molecular formula for each:
| Carbons in longest chain | Alkanes | Alkenes | Alcohols |
| 1 | methane, CH₄ | - | methanol, CH₄O |
| 2 | ethane, C₂H₆ | ethene, C₂H₄ | ethanol, C₂H₆O |
| 3 | propane, C₃H₈ | propene, C₃H₆ | propanol, C₃H₈O |
| 4 | butane, C₄H₁₀ | butene, C₄H₈ | butanol, C₄H₁₀O |
Naming branched alkanes and alkenes
The naming conventions for organic molecules cover more than the straight chain molecules. Branched molecules are named depending on the number of carbon atoms in the branch. A branch with 1 carbon is called “methyl” and a branch with 2 carbons is called “ethyl”. This is similar to the conventions covered above, plus the “-yl-” bit just says it is a branch.
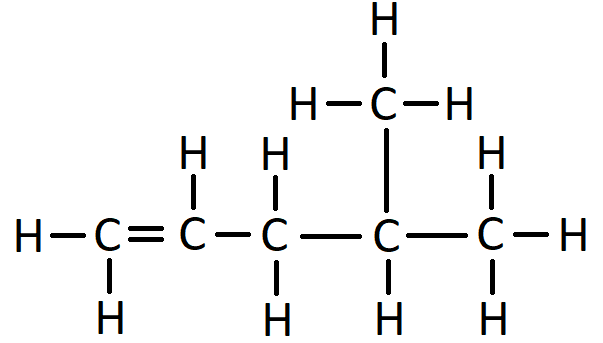
For example, this is the displayed formula for 2-methyl hexane:

In the name 2-methyl hexane, the number 2 indicates that when counting along the longest carbon chain the methyl branch comes off the second carbon atom. The “methyl” bit of the name says there is one branch of 1 carbon. The “hex” bit of the name says the longest consecutive chain of carbon atoms is 6. The “ane” bit says the molecule has only single bonds.
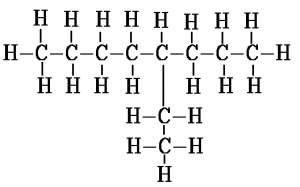
When counting along the carbon atoms of the longest chain to work out the name, the numbering of carbon atoms starts from the end nearest to the branch. Another way to put this is that the name is given such that the numbers in the name are as low as possible. For example, here is the displayed formula for 4-ethyl octane:
Another example, this time with 2 methyl branches coming off the second and third carbons of the chain, is 2,3-dimethyl hexane. The “di” in the name indicates there are two methyl groups. This is the same way in which “di” indicates there are two oxygen atoms in carbon dioxide.

Another example of how the naming convention works for branches is 2,2-dimethyl hexane:
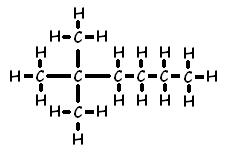
This naming of branches also applies to alkenes. Here is the displayed formula of 4-methylpent-1-ene:
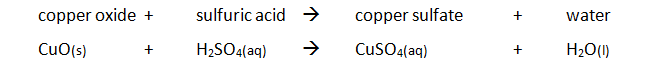
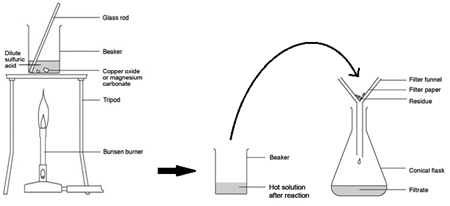
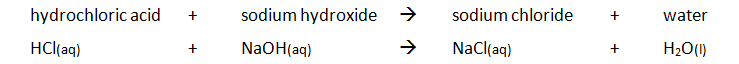
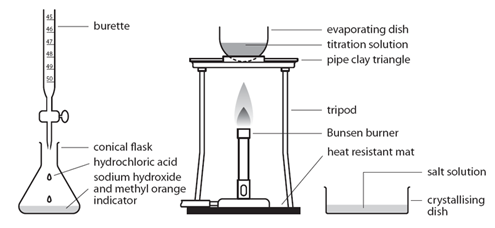
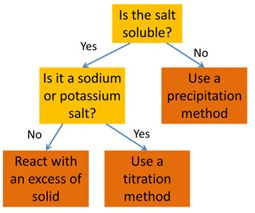
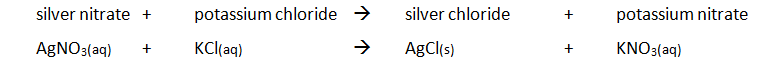
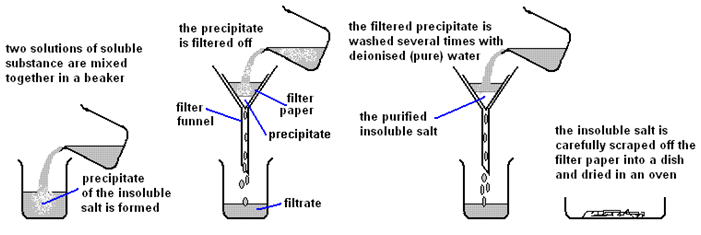


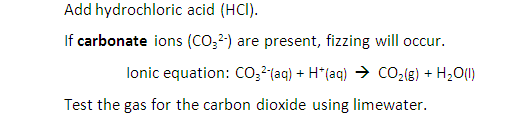


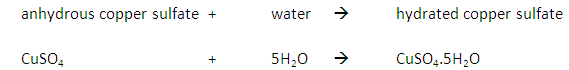
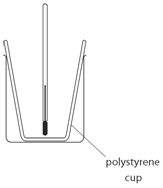
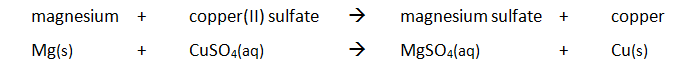

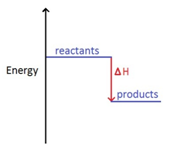
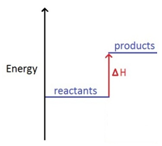
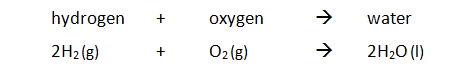
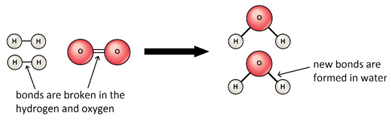
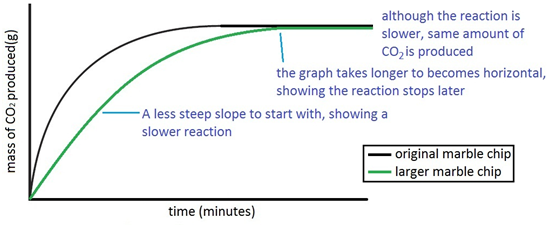
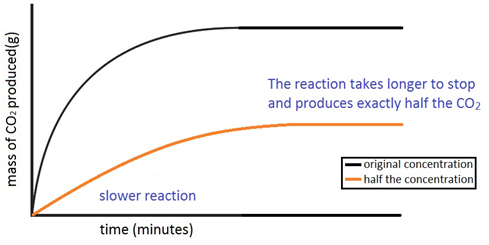
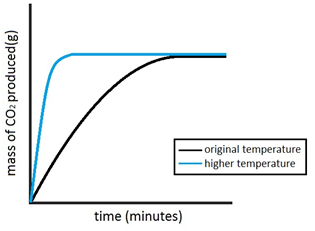
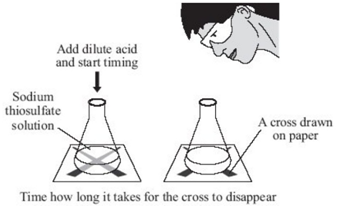
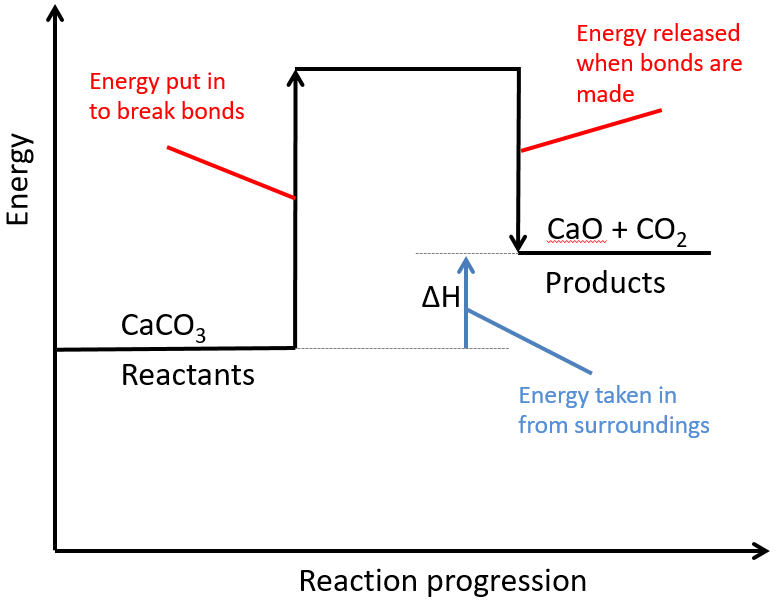
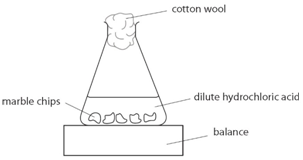 Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.
Marble chips, calcium carbonate (CaCO3) react with hydrochloric acid (HCl) to produce carbon dioxide gas. Calcium chloride solution is also formed.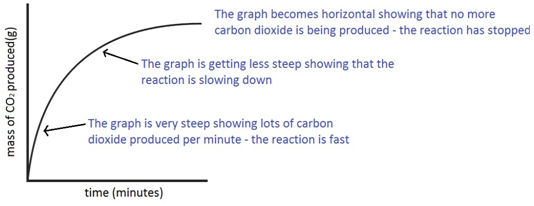
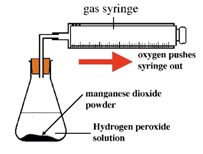
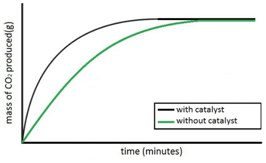
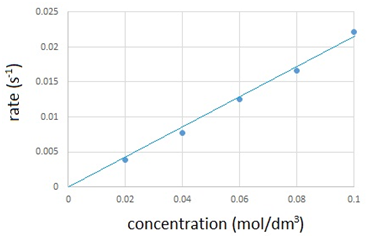
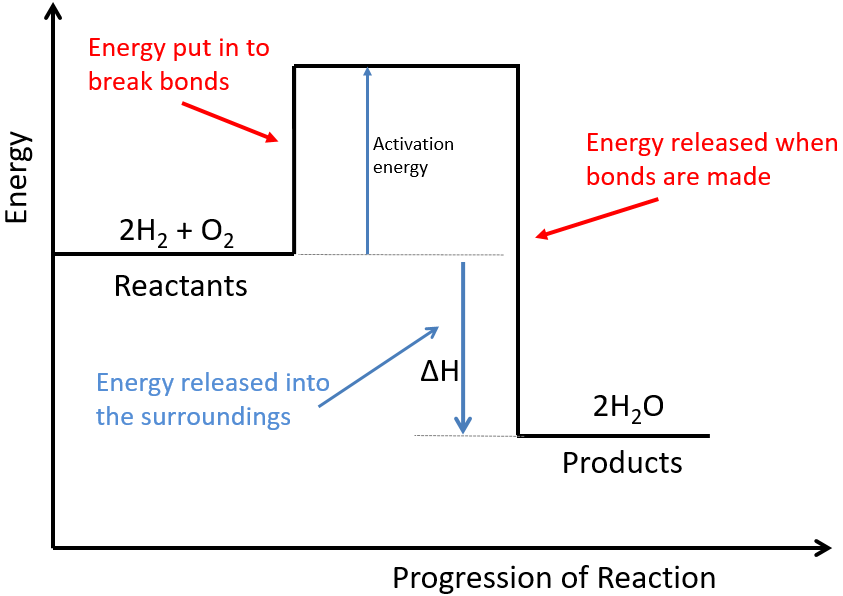
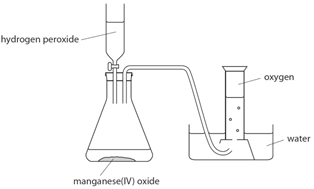 Oxygen (O2) is made in the lab from hydrogen peroxide (H2O2) using manganese(IV) oxide (MnO2) as a catalyst.
Oxygen (O2) is made in the lab from hydrogen peroxide (H2O2) using manganese(IV) oxide (MnO2) as a catalyst.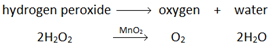
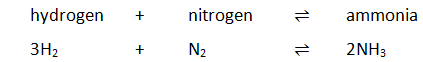
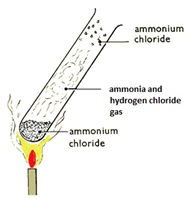 Heating ammonium chloride
Heating ammonium chloride