6.20 know and use the relationship: input power = output power V_p I_p=V_s I_p for 100% efficiency

the units for:
frequency of decay : becquerel (Bq), 1 (Bq) for 1 decay / sec
distance : centimetres (cm), normally however is (m)
time : hour (h), minute (min) but normally (s)
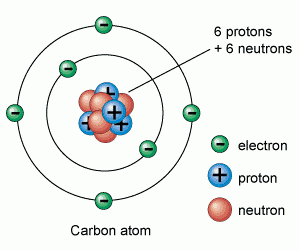
Atoms are made up of protons, neutrons and electrons.
Protons and neutrons are in the nucleus, electrons are in the shells
|
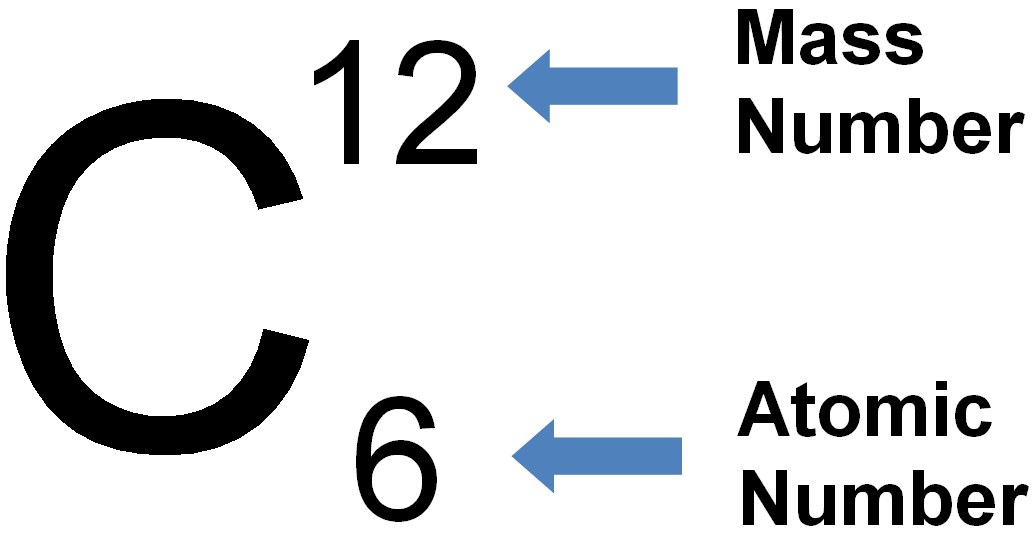
Atomic (proton) number is the number of protons in the nucleus of an atom. Mass (nucleon) number is the total number of protons and neutrons in the nucleus of an atom. An isotope is an atom of the same element, i.e. it has the same number of protons/same atomic number, but has a different number of neutrons/different mass number. Two atoms with the same atomic number but different mass numbers are isotopes see 7.02 for example |
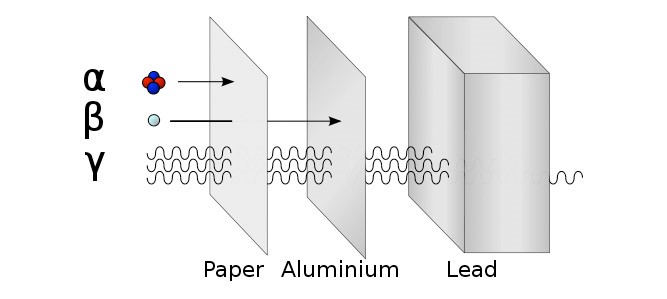
There are three types of ionising radiation:
Alpha (α), Beta (β) and Gamma (γ)
One radioactive source can release different types of radiation.
Ionisation is when an atom loses or gains an electron, causing it to become an ion (an atom which is positively or negatively charged).
|
Detect using a Geiger Müller Tube. Try the three different materials in order, paper then aluminium then lead. Count rate will significantly decrease if radiation is stopped. |
|
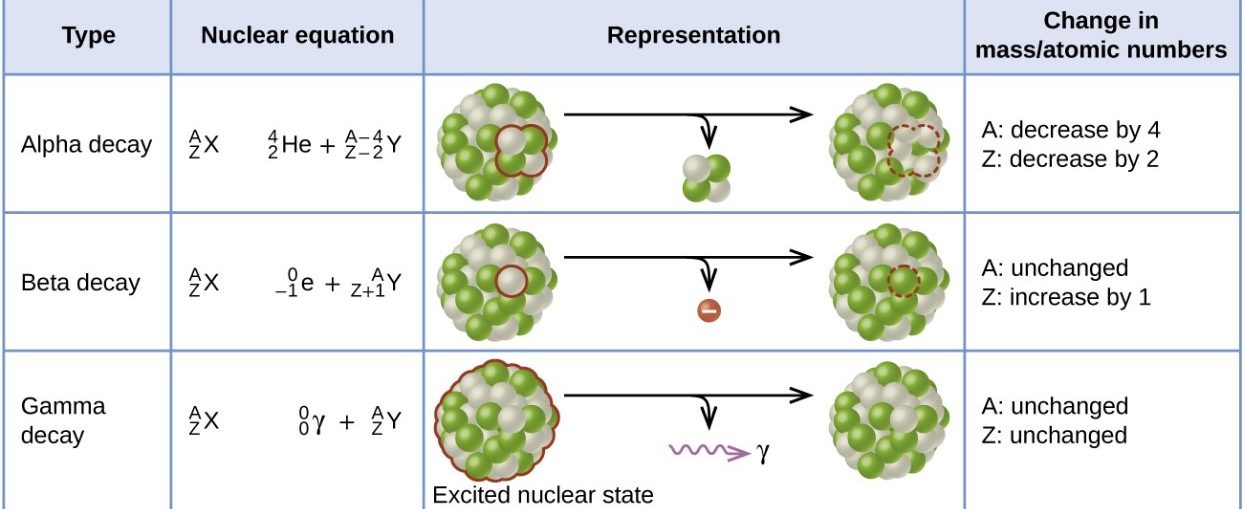
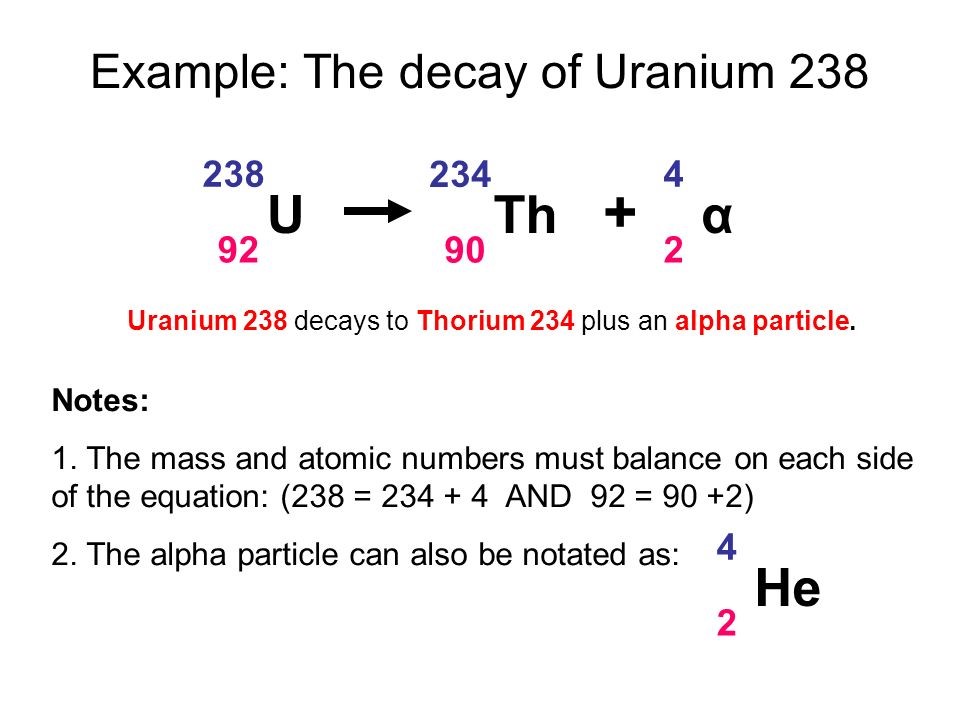
Alpha decay: · 2 protons and 2 neutrons are lost. · Mass number decreases by 4 · Atomic number decreases by 2 Beta decay · 1 neutron is converted to an electron (lost from the atom) and proton · Mass number is unchanged · Atomic number increases by 1 Gamma decay · Energy is lost from an atom in the form of an electromagnetic wave · Mass number is unchanged · Atomic number is unchanged |
|
Particle |
Symbol |
Mass |
Charge |
|
Alpha |
α |
4 |
+2 |
|
Beta |
β |
0 |
-1 |
|
Gamma |
γ |
0 |
0 |
Geiger Müller detector:
When connected to a counter, the detector will be able to measure radioactivity.
Photographic film:
Radiation will cause photographic film to darken.
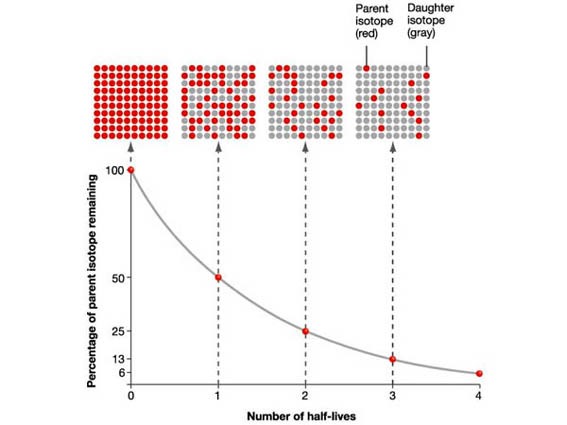
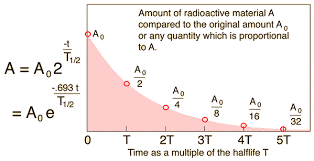
The activity of a radioactive source decreases over a period of time and is
measured in becquerels.
The Half-life is the time taken for the radioactivity of a specific isotope to fall to half its original value.
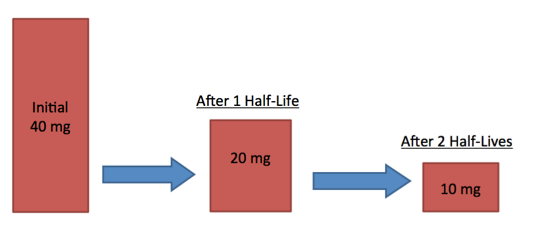
Numerical:
A radioactive source has a half-life of 2 hours. If the mass starts at 40mg, what will the mass be after 4 hours?
Graphical:
Several different times for the half-life can be calculated and averaged.
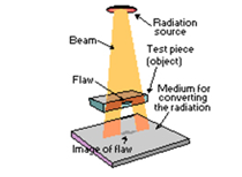
Gamma radiography:
|
Medical tracer: – Radioactive tracer put in body (swallowed/injected) – Detector put around body – Computer generates an image |

|
Gauging: – Coal absorbs a lot of radiation – If only a small amount of radiation is detected back after it is reflected by what you are trying to gauge, lots of coal is present. |

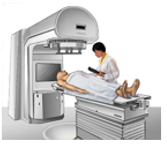
Radiotherapy
|
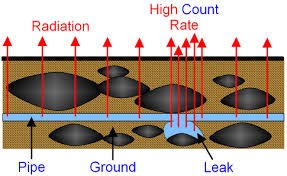
Pipe tracers: – A radioactive material which emits gamma radiation with a short half-life is put in the water – A detector is placed above the pipe – A spike in detected radioactivity suggests a leak in the pipe |
Sterilisation:

Carbon dating:
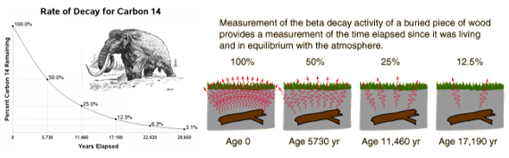
Occurs when material that contains radioactive atoms is deposited on materials, skin, clothing, or any place where it is not desired.
The process by which an object is exposed to radiation.
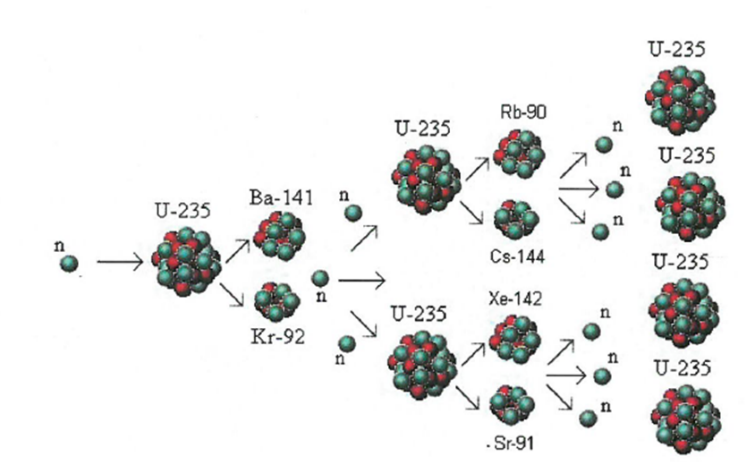
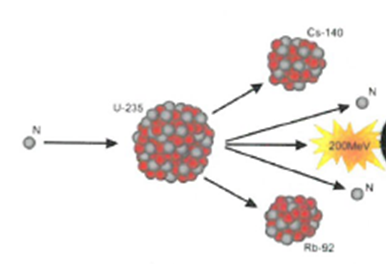
The process where heavy atoms are split into smaller, lighter atoms. This releases energy.
The process where lighter atoms are forced to join together to make heavier atoms. This releases energy.
Within the core of the Earth, radioactive isotopes of elements such as uranium, thorium and potassium provide a large proportion of the heat within the Earth through radioactive decay.
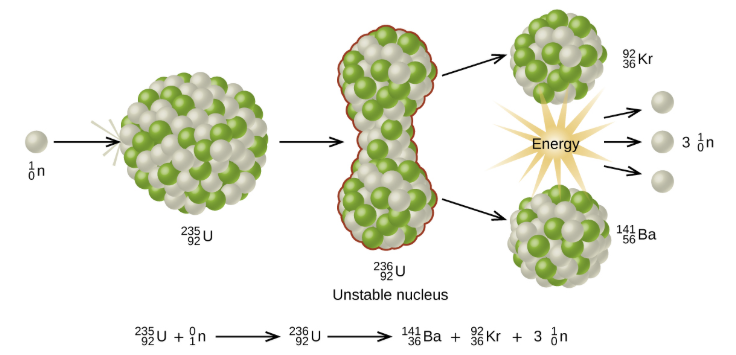
Chain Reaction:
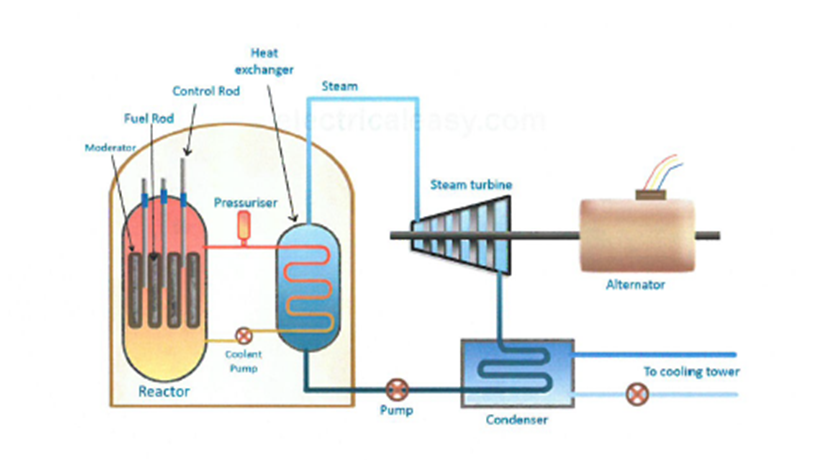
Moderator:
Control rods:
Shielding:
Fission – Larger nuclei are split into smaller nuclei.
Fusion – Two smaller nuclei collide and combine to form a larger nucleus.
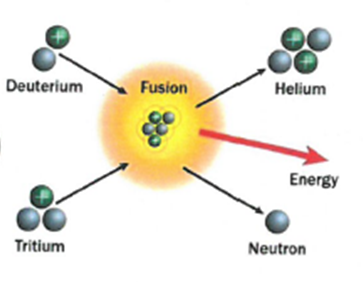
Fusion:
units for:
Mass: kilogram (kg)
distance: metre (m)
velocity: metre per second (m/s)
acceleration: metre per second squared (m/s2)
Force: newton (N)
time: second (s)
gravitational field strength: newton/kilogram (N/kg)

The Milky Way galaxy contains billions of stars
The Universe – billions of galaxies
|
An object’s gravitational field strength depends on its MASS. A massive object, like a star, will have a very large g-field. The Moon has less mass than the Earth, so its gravitational field is much weaker – approx 1/6th of the Earth’s. This means that we could jump higher on the Moon, and objects would fall more slowly, as they experience a weaker gravitational force.
A planet with a large radius will have a weaker gravitational field at its surface, because the surface is further away from the centre of the planet. |
According to Newton, there is an attractive gravitational force between any two objects– pulling them together. E.g. the planets and comets experience an attractive force towards the Sun.
Moons and artificial satellites are attracted to their planets, and so are pulled towards them.
This gravitational force keeps them moving in curved paths called orbits. The Moon does not crash into the Earth, and the planets do not crash into the Sun because they are moving.
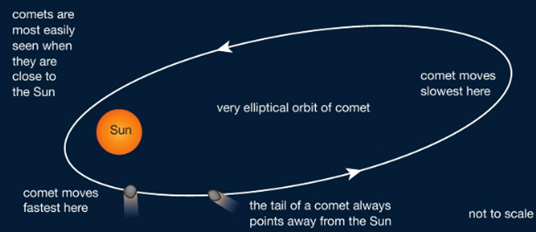
Comets have highly elliptical orbits, with the Sun at one focus. When they come in close to the Sun they speed up, due to the larger gravitational force on them. They also develop bright tails that point away from the centre of the Sun. These are caused by tiny ice crystals that melt and break off from the comet and reflect the bright light of the Sun.
Moons have circular orbits and planets orbit in slightly squashed circles, called ellipses.
|
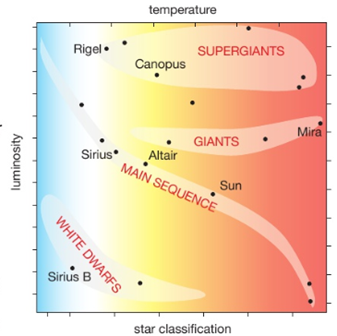
Stars are classified into 7 groups according to their colours (which is due to their surface temperature); O,B,A,F,G,K,M O are hottest (> 33 000 K and blue), M are coolest (2000 – 3700K and red) |
|
• nebula Stars form from large clouds of dust and gas particles (nebulae) that are drawn together by gravitational forces over millions of years. As the particles get closer the temperature and pressure becomes so large that nuclear fusion of hydrogen nuclei to helium nuclei occurs. This releases enormous amounts of energy in the form of heat and light.
• star (main sequence) Fusion produces forces that make the star expand outwards, but gravitational force is always pulling the particles within the star inwards. When these two opposing forces become balanced a star is stable and called a main sequence star. It should stay this way for millions of years, at a constant size and temperature.
• red giant Eventually hydrogen fusion stops as the star runs out of fuel. Gravitational force is now bigger than the outward fusion force which causes the star to collapse inwards and compress. This causes it to heat up to even higher temperatures so that fusion of helium nuclei begins. The increased power output causes the star to expand greatly. The surface area is so large that it is cooler than before, so its colour changes to red and the star is called a red giant.
• white dwarf Eventually fusion stops when the star runs out of helium nuclei and the gravitational force causes the star to collapse inwards and compress again. This heats it up so it changes colour to emit white light. The star is squashed so greatly by the gravitational force to become a small and very dense white dwarf. (They are so dense that a teaspoon full would weigh more than a cruise liner). A white dwarf eventually cools down and change colour as it does so, eventually becoming black. |
After the stable period, a giant star expands into red supergiant. (It produces all the elements up to iron during nuclear fusion). When it finally runs out of nuclei to fuse it collapses due to the gravitational force, and then explodes – an exploding star is called a supernova.
The explosion throws dust and gas back into space and so another nebula is formed. A dense core remains – called a neutron star, because it is made entirely from neutrons. If its mass is large enough it can compress further to become a black hole. (Their gravity is so strong that not even light can escape!)
Apparent magnitude is simply how bright a star appears in the night sky.
But, the brightness of a star depends on its distance from Earth and its luminosity – how much power it produces. (A dim star could just be very far away, or very close but not very luminous).
Absolute magnitude enables us to compare the brightness of stars because it is a measure of how bright they would appear if they were all the same distance from the Earth. – 32.6 light years.
This diagram shows the relationship between a star’s luminosity (its brightness or power output) and its surface temperature. A star moves to different positions in the diagram during its life, as its internal structure and temperature change.
Important Note: the temperature scale is reversed. It is hotter towards the origin of the x axis.
Scientists believe that about 14 billion years ago all matter in the Universe was in one extremely tiny and dense place. It then suddenly exploded, and has been expanding ever since. This expansion is shown by the red-shift of galaxies and the energy of the explosion can be seen everywhere in the Universe as the CMBR.
|
If we examine the light spectra for distant galaxies we can see that the wavelengths of the light have become longer. We call this stretching of the waves ‘red-shift’. It tells us that the galaxies producing the light are moving away from us .The further away a galaxy is, the greater its red-shift, so it is moving even faster. This is evidence that the Universe is expanding and so it supports the Big Bang Theory.
Microwave radiation can be detected EVERYWHERE in the Universe. These are the stretched remains of high energy gamma radiation that would have been produced in the explosion that created the Universe. They have stretched because the Universe is expanding. |
|
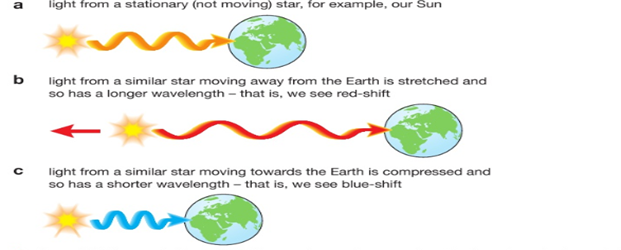
This is called the Doppler Effect. If something that emits a wave moves whilst it is doing so (imagine a noisy motorbike coming towards you then going further away, emitting sound waves the whole time) then the wavelength of the sound will become shorter as it is moving towards you, increasing the frequency, and stretched as it is moving away, decreasing the frequency.
You will hear this as a change in pitch, getting higher as it approaches and lower as it moves away. The same thing happens for a moving object that is emitting light waves –e.g. a galaxy. |
The further away a galaxy is the greater its red-shift and hence the faster it is moving away from us.
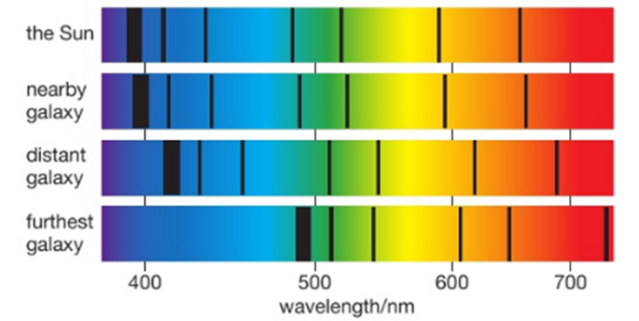
More distant galaxies have greater red-shift and therefore are receding faster because the space between all galaxies is stretching as the universe expands.