5.22 use the relationship between the pressure and volume of a fixed mass of gas at constant temperature:
P1V1 = P2V2
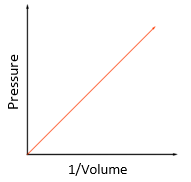
Boyle’s law:
For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.


P1V1 = P2V2
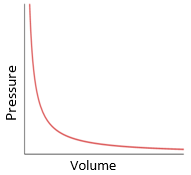
Boyle’s law:
For a fixed mass of gas at constant temperature, the pressure is inversely proportional to the volume.
The unit for:
Current : amps (A)
Potential Difference : volt (V)
power : watt (W)
Opposites attract: North attracts South and South attracts North
Like charges repel: Two Norths will repel each other
Permanent magnets are made of magnetically hard materials such as steel. These materials retain their magnetism once magnetised.
Some materials like iron are magnetically soft. They lose their magnetism once they are no longer exposed to a magnetic field. They are used as temporary magnets such as electromagnets.
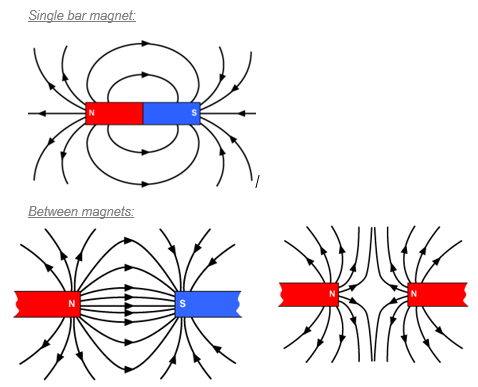
Around every magnet there is a region of space where we can detect magnetism (where magnetic materials will be affected).
This is called the magnetic field and in a diagram we represent this with magnetic field lines.
The magnetic field lines should always point from north to south.
|
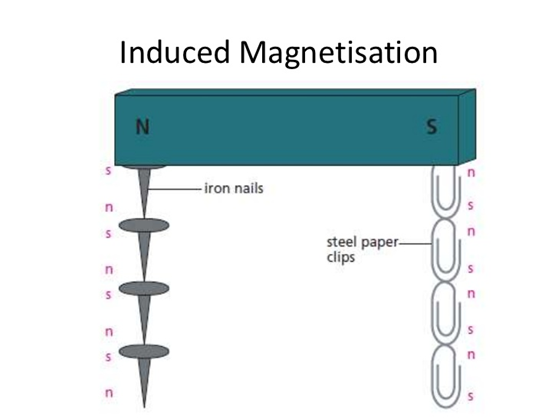
When magnetic materials are bought near or touch the pole of a strong or permanent magnet, they become magnets. This magnetic character is induced in the objects and it is removed when the permanent magnet is removed. This is a temporary magnet Magnetism is induced in the paperclips so each paperclip can attract another one |
|
A uniform magnetic field is comprised of straight, parallel lines which are evenly spaced. Between two opposite charges on flat magnets, a uniform magnetic field is formed. |
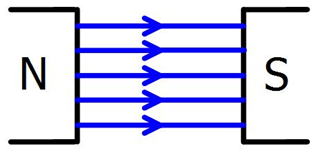
|
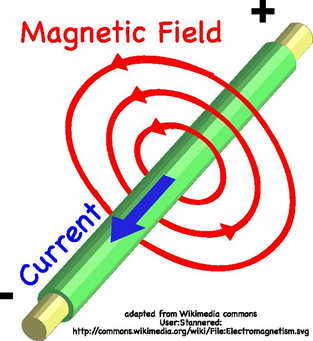
A current travelling along a wire produces a circular magnetic field around the wire. The magnetic field direction can be determined using the right hand grip rule. |
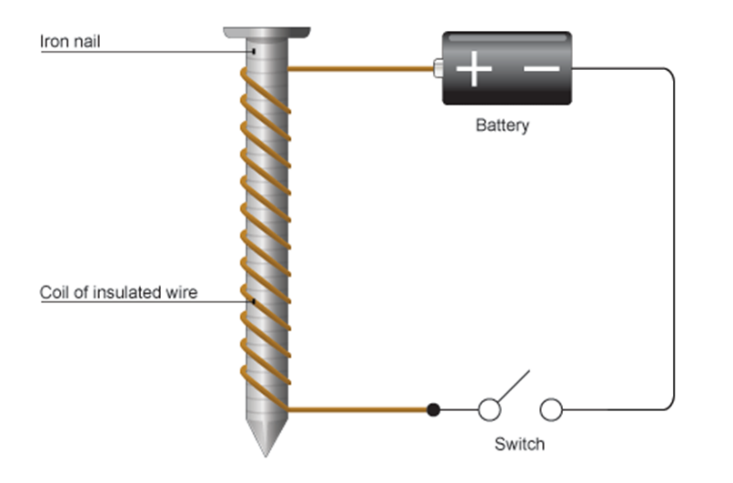
A soft iron core wrapped in wire. When current flows through the coil of wire it becomes magnetic.
|
The movement of the charged particle is a current so it produces a magnetic field. This magnetic field interacts with the permanent magnetic field to create a force. The force is perpendicular to the direction of motion and the permanent magnetic field. |
Motor
Loudspeaker
|
Fleming’s left hand rule. Thumb: force First finger: Magnetic Field Second finger: Current |
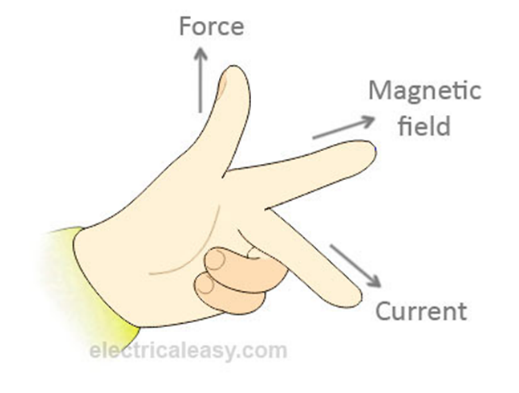
|
If you increase the magnitude of the current through a wire or the size of the magnet being used, you increase the force on the wire. If you change the direction of the current or reverse the poles of the magnet, you change the direction of the force on the wire |
When a conductor (can be a wire, coil or just a piece of metal) experiences a changing magnetic field a potential difference or voltage is induced in it. The strength of the potential difference depends on the strength of the magnetic field, how fast it changes i.e. how fast the coil is spinning, and how much of the conductor is exposed to the field i.e. how many turns in the coil.
|
Electricity can be generated by either moving a magnet inside a coil of wire or rotating a coil inside a permanent magnetic field.
Model answer for a generator (Rotating coil): · Coil is rotated within a magnetic field · As it turns the coil cuts the magnetic field lines. · This induces a voltage (or current) in the coil. · This can then be connected to an existing circuit. · In a generator, energy is being converted from kinetic (mechanical) energy into electrical energy. · The size of the induced voltage (or current) can be increased by: · Using a stronger magnet · Having more turns in the coil · Spinning/moving the coil faster.
Model answer for a generator (Rotating magnet) · Magnet is rotated within a coil · As it turns the coil cuts the constantly changing magnetic field lines from the magnet. · This induces a voltage (or current) in the coil. · This can then be connected to an existing circuit. · In a generator, energy is being converted from kinetic (mechanical) energy into electrical energy. · The size of the induced voltage (or current) can be increased by: · Using a stronger magnet · Having more turns in the coil · Spinning/moving the magnet faster.
|
|
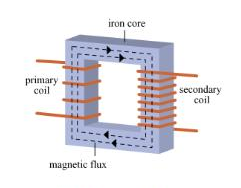
AC current in the primary coil produces a changing magnetic field around the primary coil. The iron core channels the changing field through the secondary coil. The changing magnetic field induces a voltage in the secondary coil. |
|
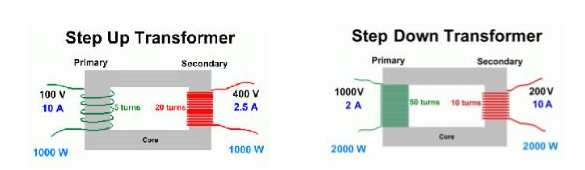
Step Up transformers increase the voltage – more secondary turns than primary Step Down transformers decrease the voltage – more primary turns than secondary |
the units for:
frequency of decay : becquerel (Bq), 1 (Bq) for 1 decay / sec
distance : centimetres (cm), normally however is (m)
time : hour (h), minute (min) but normally (s)
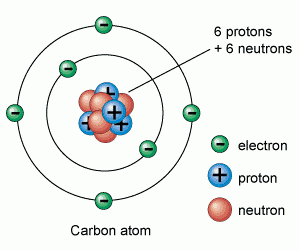
Atoms are made up of protons, neutrons and electrons.
Protons and neutrons are in the nucleus, electrons are in the shells
|
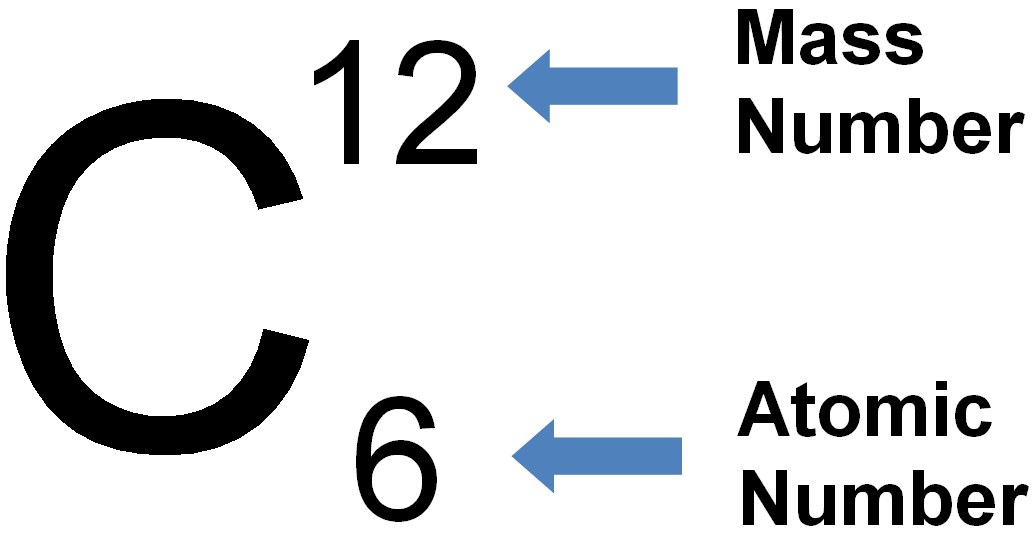
Atomic (proton) number is the number of protons in the nucleus of an atom. Mass (nucleon) number is the total number of protons and neutrons in the nucleus of an atom. An isotope is an atom of the same element, i.e. it has the same number of protons/same atomic number, but has a different number of neutrons/different mass number. Two atoms with the same atomic number but different mass numbers are isotopes see 7.02 for example |
There are three types of ionising radiation:
Alpha (α), Beta (β) and Gamma (γ)
One radioactive source can release different types of radiation.
Ionisation is when an atom loses or gains an electron, causing it to become an ion (an atom which is positively or negatively charged).
|
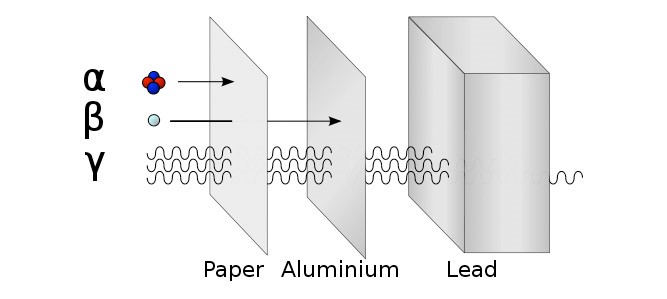
Detect using a Geiger Müller Tube. Try the three different materials in order, paper then aluminium then lead. Count rate will significantly decrease if radiation is stopped. |
|
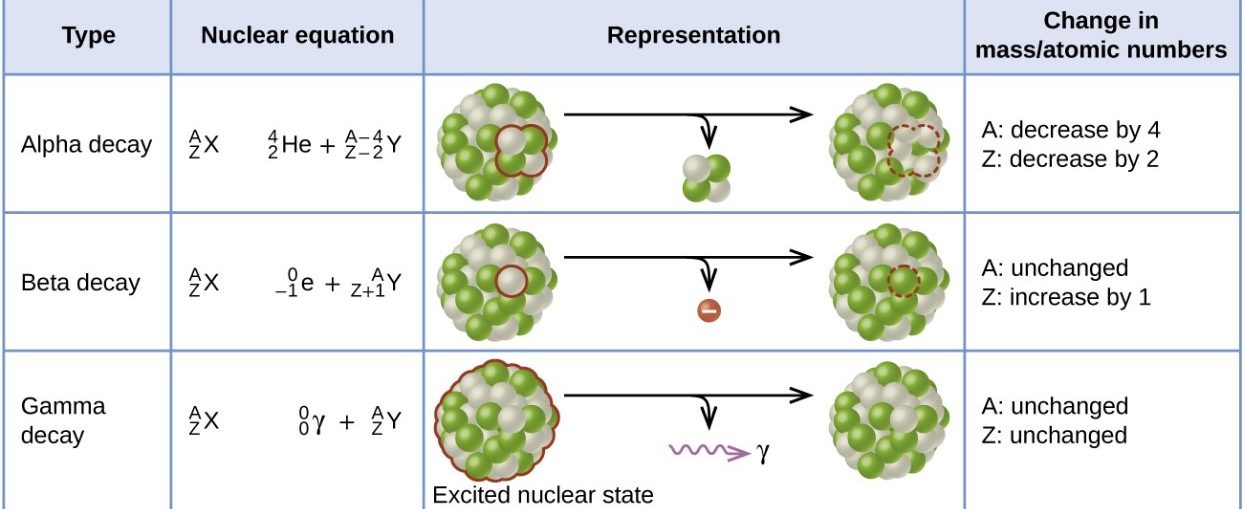
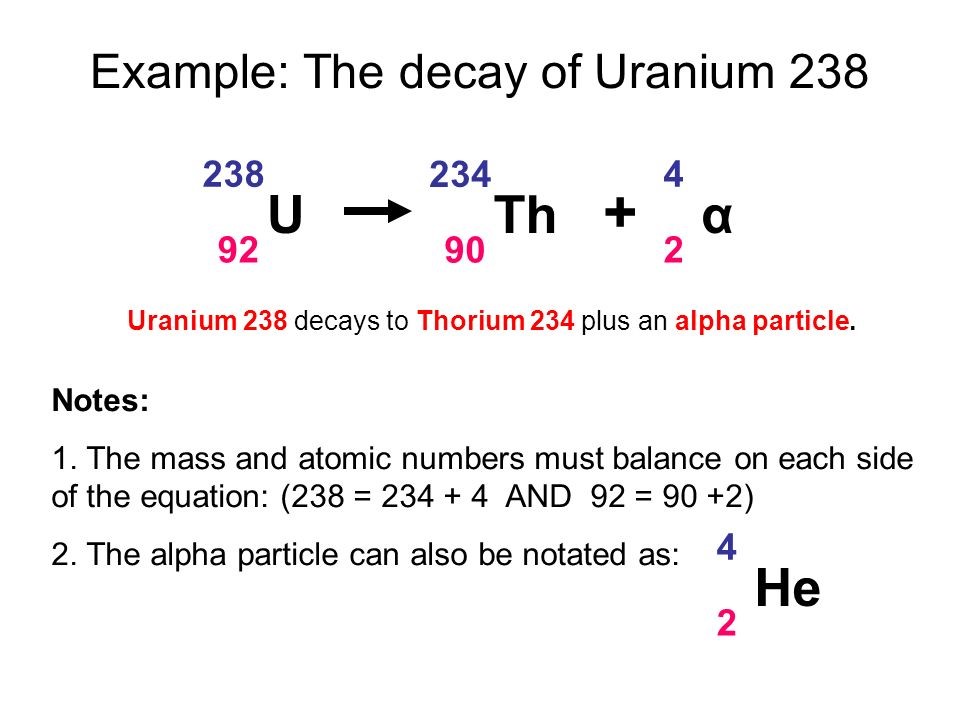
Alpha decay: · 2 protons and 2 neutrons are lost. · Mass number decreases by 4 · Atomic number decreases by 2 Beta decay · 1 neutron is converted to an electron (lost from the atom) and proton · Mass number is unchanged · Atomic number increases by 1 Gamma decay · Energy is lost from an atom in the form of an electromagnetic wave · Mass number is unchanged · Atomic number is unchanged |
|
Particle |
Symbol |
Mass |
Charge |
|
Alpha |
α |
4 |
+2 |
|
Beta |
β |
0 |
-1 |
|
Gamma |
γ |
0 |
0 |
Geiger Müller detector:
When connected to a counter, the detector will be able to measure radioactivity.
Photographic film:
Radiation will cause photographic film to darken.
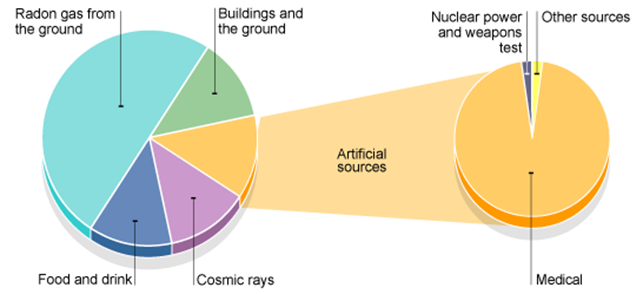
The activity of a radioactive source decreases over a period of time and is
measured in becquerels.
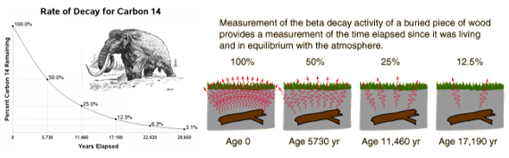
The Half-life is the time taken for the radioactivity of a specific isotope to fall to half its original value.
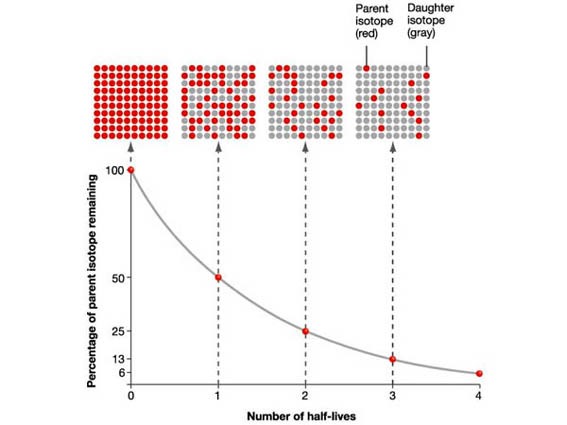
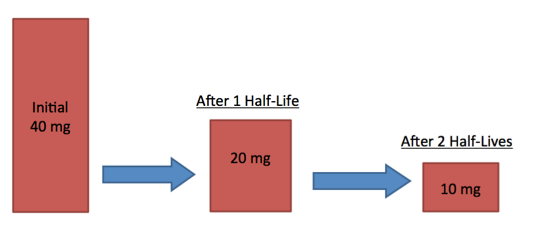
Numerical:
A radioactive source has a half-life of 2 hours. If the mass starts at 40mg, what will the mass be after 4 hours?
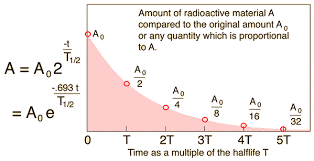
Graphical:
Several different times for the half-life can be calculated and averaged.
Gamma radiography:
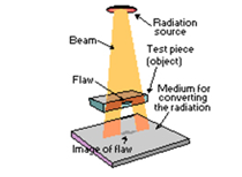
|
Medical tracer: – Radioactive tracer put in body (swallowed/injected) – Detector put around body – Computer generates an image |

|
Gauging: – Coal absorbs a lot of radiation – If only a small amount of radiation is detected back after it is reflected by what you are trying to gauge, lots of coal is present. |

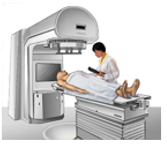
Radiotherapy
|
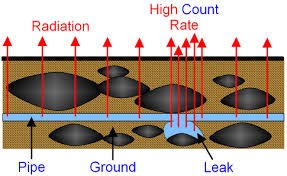
Pipe tracers: – A radioactive material which emits gamma radiation with a short half-life is put in the water – A detector is placed above the pipe – A spike in detected radioactivity suggests a leak in the pipe |
Sterilisation:

Carbon dating:
Occurs when material that contains radioactive atoms is deposited on materials, skin, clothing, or any place where it is not desired.
The process by which an object is exposed to radiation.
The process where heavy atoms are split into smaller, lighter atoms. This releases energy.
The process where lighter atoms are forced to join together to make heavier atoms. This releases energy.
Within the core of the Earth, radioactive isotopes of elements such as uranium, thorium and potassium provide a large proportion of the heat within the Earth through radioactive decay.
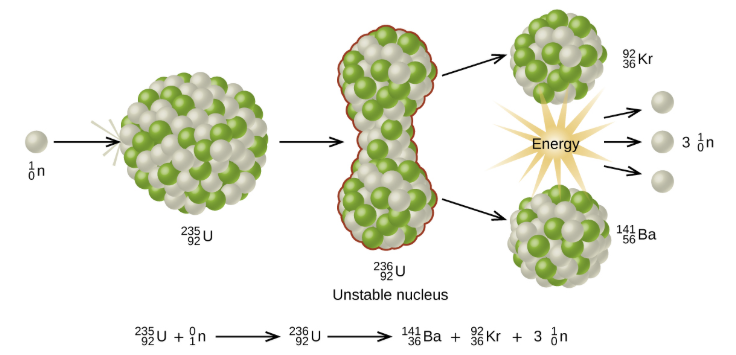
Chain Reaction:
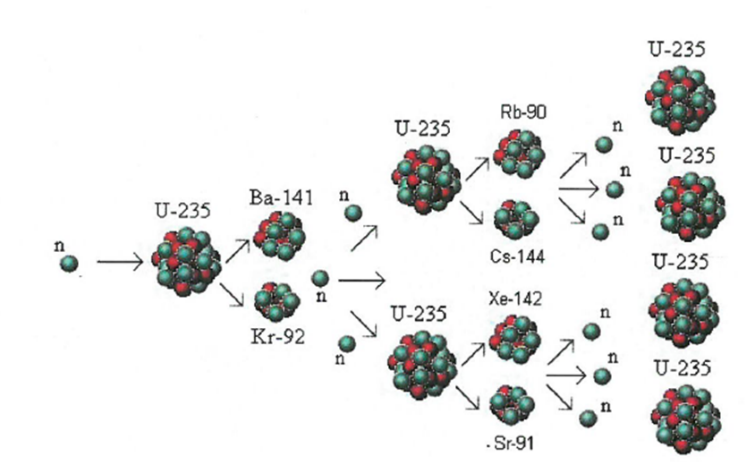
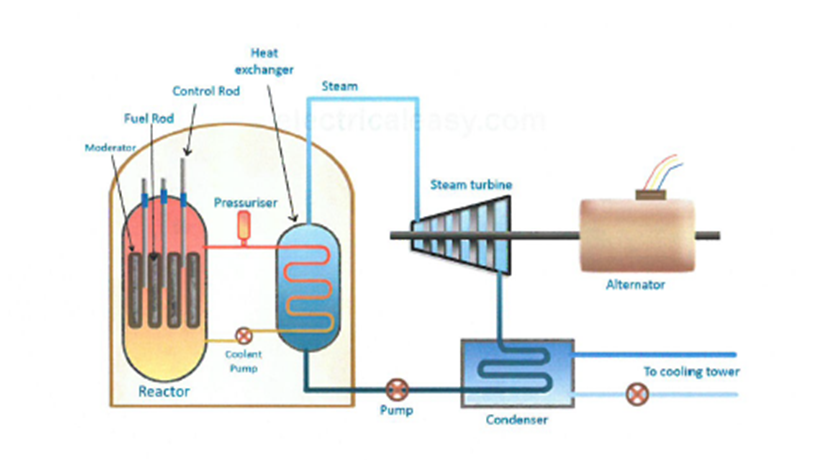
Moderator:
Control rods:
Shielding:
Fission – Larger nuclei are split into smaller nuclei.
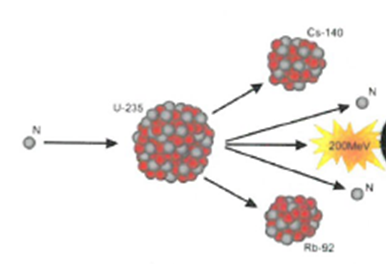
Fusion – Two smaller nuclei collide and combine to form a larger nucleus.
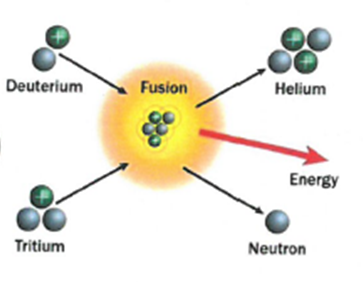
Fusion:
units for:
Mass: kilogram (kg)
distance: metre (m)
velocity: metre per second (m/s)
acceleration: metre per second squared (m/s2)
Force: newton (N)
time: second (s)
gravitational field strength: newton/kilogram (N/kg)

The Milky Way galaxy contains billions of stars
The Universe – billions of galaxies
|
An object’s gravitational field strength depends on its MASS. A massive object, like a star, will have a very large g-field. The Moon has less mass than the Earth, so its gravitational field is much weaker – approx 1/6th of the Earth’s. This means that we could jump higher on the Moon, and objects would fall more slowly, as they experience a weaker gravitational force.
A planet with a large radius will have a weaker gravitational field at its surface, because the surface is further away from the centre of the planet. |