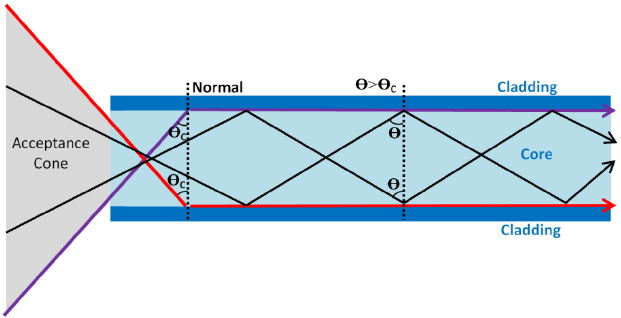
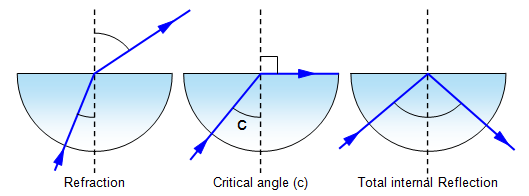
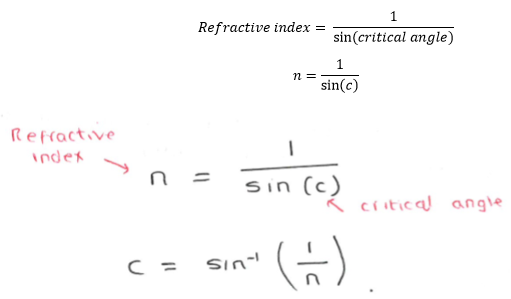
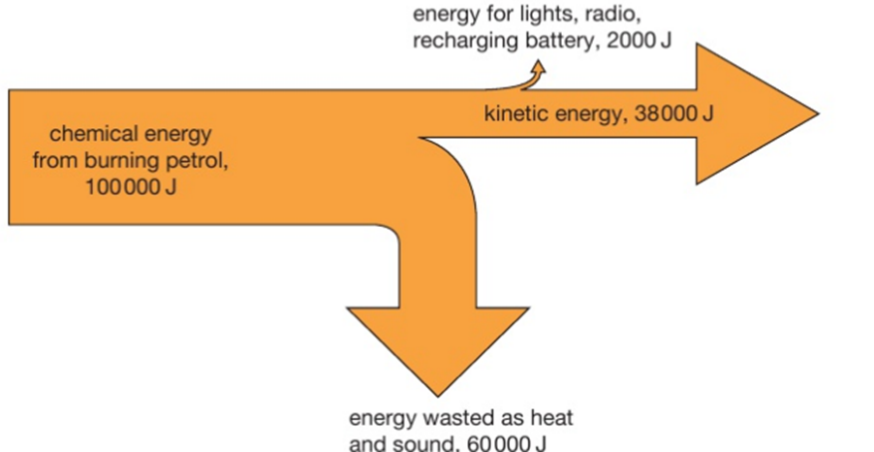
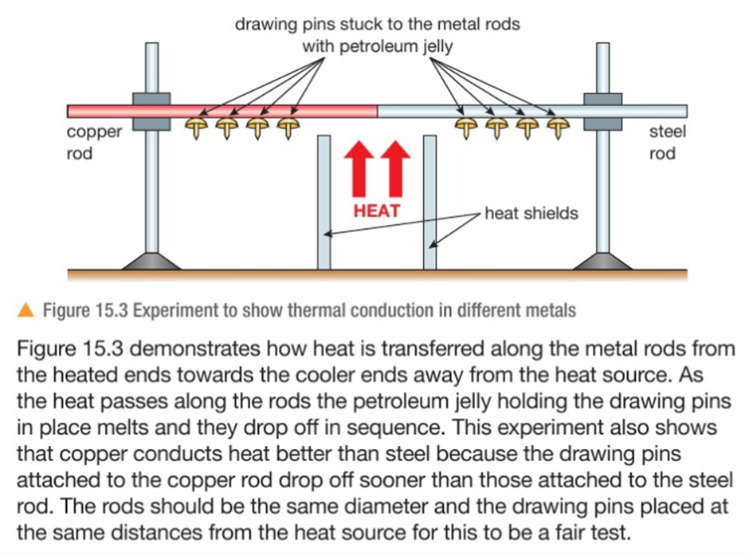
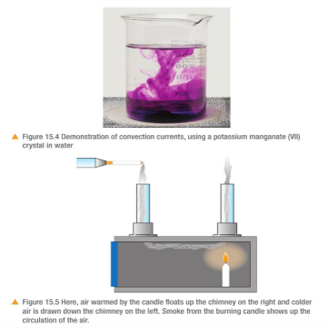
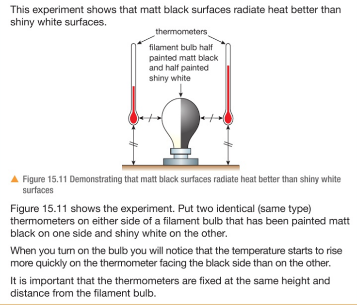
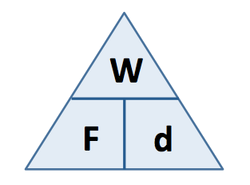
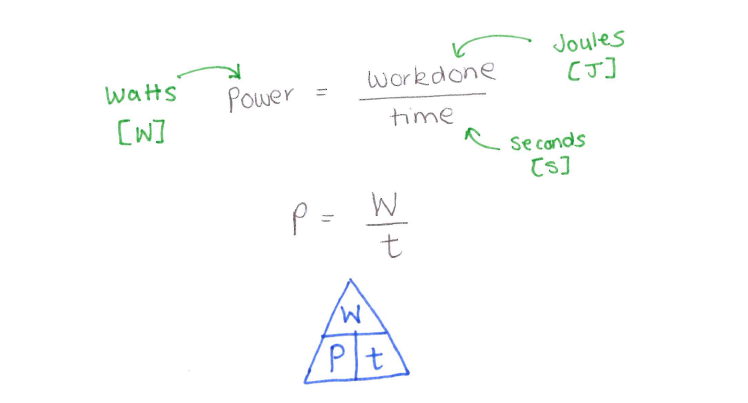
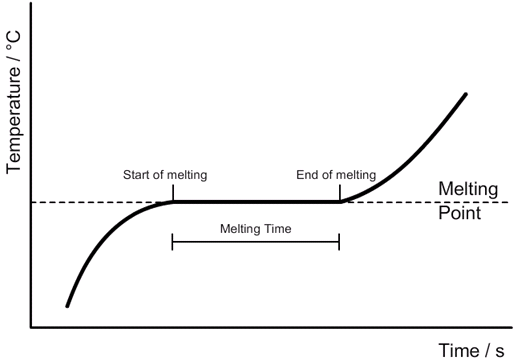
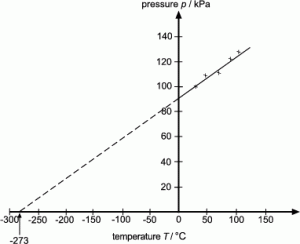
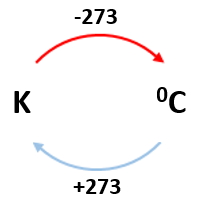
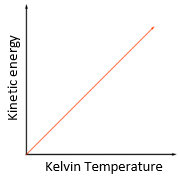
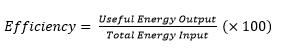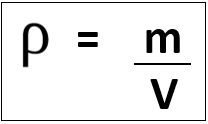3.19 practical: investigate the refractive index of glass, using a glass block
|
1. Set up your apparatus as shown in the diagram using a rectangular block. 2. Shine the light ray through the glass block 3. Use crosses to mark the path of the ray. 4. Join up crosses with a ruler 5. Draw on a normal where the ray enters the glass block 6. Measure the angle of incidence and the angle of refraction and add these to your results table 7. Calculate the refractive 8. Repeat steps 2 – 7 using 9. Find an average of your
|