4:08 describe how the industrial process of fractional distillation separates crude oil into fractions
- Crude oil is separated by fractional distillation.
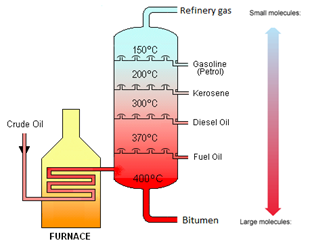
- Crude oil is heated and the oil evaporates.
- The gas goes into the fractional distillation tower. As the gas rises the temperature falls.
- Fractions with higher boiling points condense and are collected nearer the bottom of the tower.